Week 3-a
Structure and Function of Proteins \n \n \n
- Protein Structure
- The conformation(or shape) of a protein predicts its function
- There are several general types of protein structures:
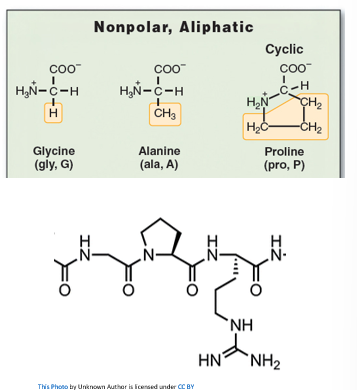
- Globular proteins
- Fibrous proteins
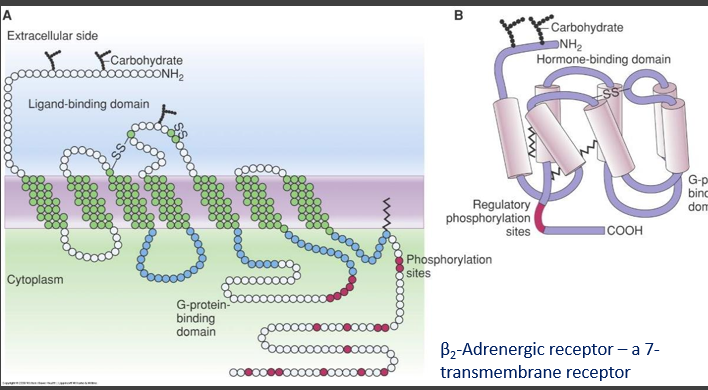
- Transmembrane proteins
- DNA binding proteins
- Bonding in proteins
- Proteins are held in their shape mostly by non-covalent bonds
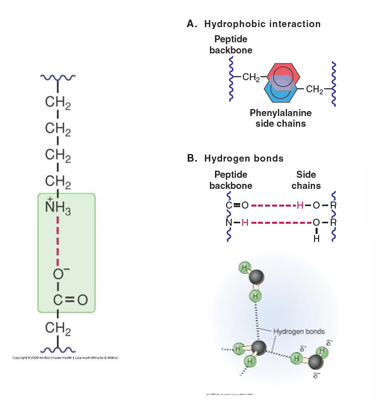
- Electrostatic (Ionic) Interactions
- Interactions between charged groups on different amino acids
- Hydrogen Bonds
- Interactions between amino acids mediated by partial charges on hydrogens
- Hydrophobic Interactions
- Grouping of non-polar amino acids
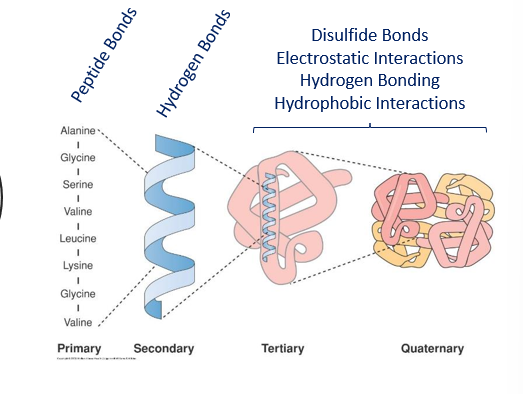
- Levels of structure
- From Amino Acids to Proteins
- How proteins fold is driven by how their polar and non-polar side chains interact.
- Protein structure Rules
- The three-dimensional structure must be flexible enough to function properly but stable enough that it will not convert to another conformation
- It must have amino acids with side groups that are compatible with the environment or environments (such as a transmembrane protein) the protein will function in
- So, polar groups on the outer surface if it will function in aqueous environments, hydrophobic groups in membrane insertion areas if it is a transmembrane protein
- Primary Structure
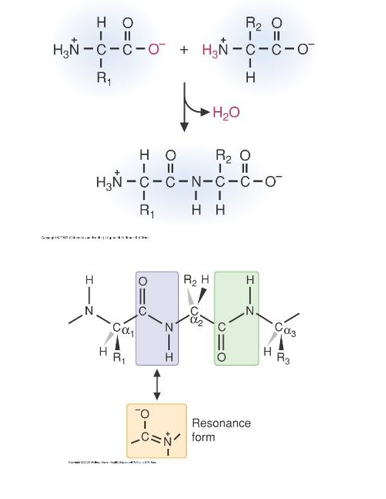
- Peptide Bonds form the backbones of proteins
- The peptide bond is between an amino group of one amino acid and a carboxyl group of another
- Because of its partial double-bond characteristics the peptide bond it is rigid
- The R-groups are usually on opposite sides of the bond
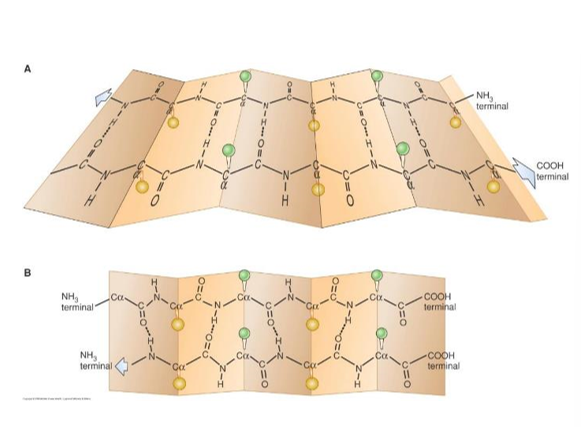
- Secondary Structure alpha-helices
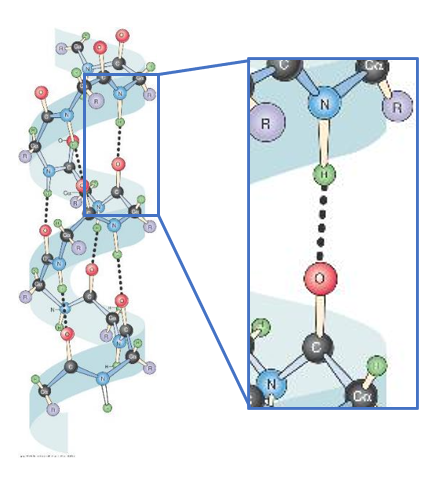
- A common secondary structure in proteins is the α-helix
- It is a regular repeating structure where the coil is maintained by Hydrogen bonds between the H in the N-H bond and the O of the carbonyl group 4 amino acid’s away in the peptide chain
- The only amino acid that is not present in an alpha-helix is proline (known as a 'helix breaker")
- This is a view from the top of an α-helix, looking down the centre
- The R-groups radiate out from the helix, this keeps them far enough apart from each other that the helix is stable
- Even though the peptide bonds are somewhat rigid, the bonds have enough ability to rotate to form an α-helix
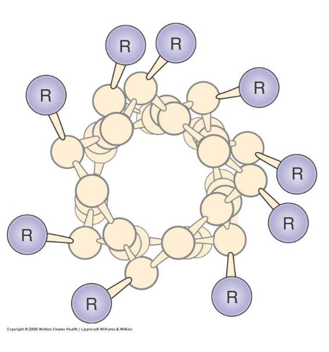
- Secondary Structure Beta-sheets
- Another type of repetitive secondary structure in proteins, again maintained by hydrogen bonding between backbone groups – not side-groups
- β-sheets can be either parallel (bottom) or anti-parallel (top), depending on whether the strands are in the same or opposite orientation
- β-sheets are more rigid structures than α-helices
- β-sheets can form “β-barrels”, structures that can transport hydrophobic substances or form pores in membranes.
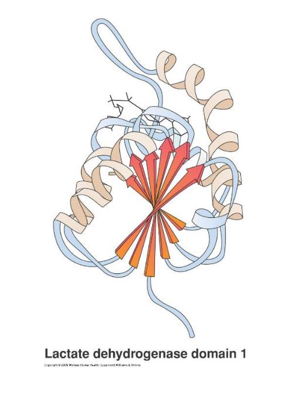
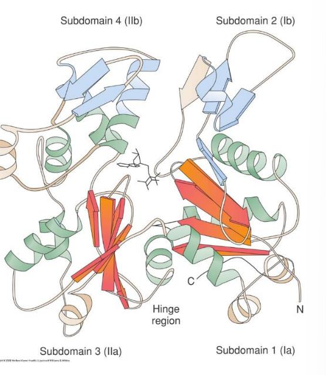
- Tertiary Structure
- The α-helices and β-sheets, combined with irregular elements such as loops and turns, make up the tertiary structure of a protein
- The amino acids in the primary structure define which secondary structure will be adopted and which tertiary structure conformation every molecule of the protein will take
- The “native conformation” of a protein is the 3-dimensional form it should take to perform its function correctly
- This is coded for in the DNA and every molecule of this protein should assume the same conformation if folded correctly
- Proteins may contain characteristic elements, called “structural domains”, that may be the similar among quite different proteins
- \n
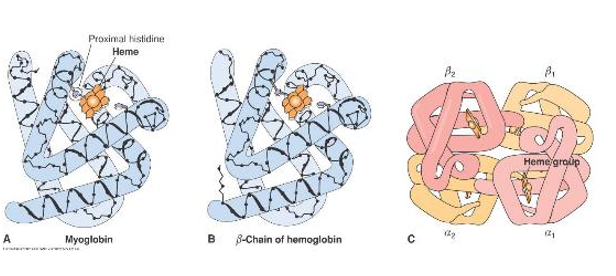
- Quaternary Structure hemoglobin
- Proteins that are made up of more than one subunit (e.g. dimers, trimers, or hemoglobin, with 4 subunits shown at right) have quaternary structure
- The subunits can be the same (such as in a homodimer) or different (such as in a heterodimer)
- In hemoglobin there are 2α-subunits and 2β-subunits
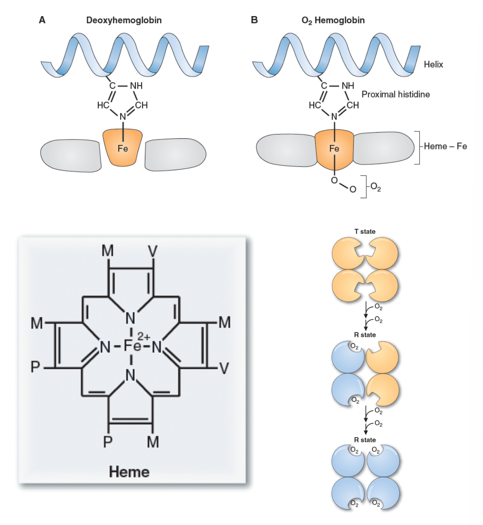
- The Fe2+ in hemoglobin can make 6-bonds
- It has 4 bonds to Heme, and 1 bond to a nearby histidine
- This leaves 1 binding slot open. This can be filled with oxygen, carbon monoxide or can remain empty
- Binding of oxygen causes a shift in the shape of hemoglobin, making it easier for more oxygen to bind to the other sites
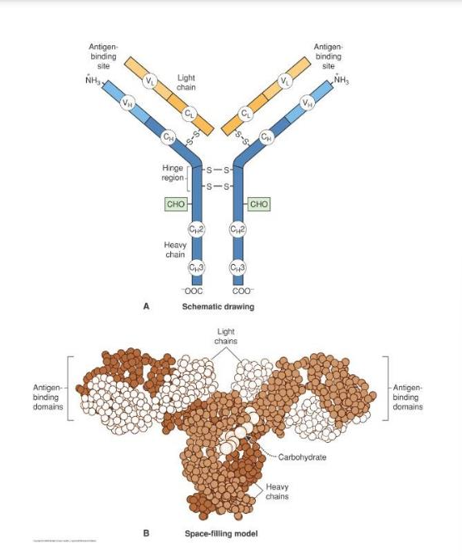
- Quaternary structure : Immunoglobins
- Antibodies, or immunoglobulins perform important defense functions in our bodies,
- The immunoglobulins all have this structure with 2 light and 2 heavy chains held together by disulphide bonds
- The antigens are bound at the ends of the “Y”, a variable region depending on the specific antibody, alerting the body to invasion
- Protein Denaturation
- Proteins need to be folded properly to function
- If a protein is unfolded or misfolded, it may be unable to carry out its role
- Proteins can be altered in several ways
- Temperature
- pH
- Protein Modifications
- Changes in temperature can impact bonding
- Change in conformation usually inhibits the function, which is why it is important to maintain proper temperature and pH
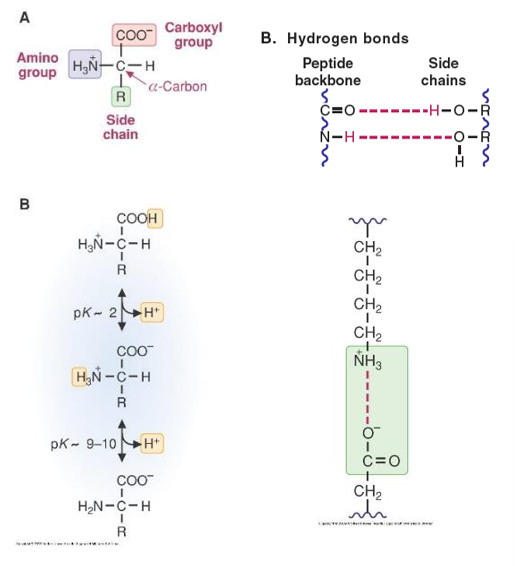
- Protonation and Deprotonation
- Changes in pH cause structural changes, due to disruption of the hydrogen and ionic bonds
- A large change in pH could cause the addition or dissociation of a proton
- Loss or gain of a proton could cause the breaking of the hydrogen bonds that hold the protein in its proper conformation
- Proteins without the correct tertiary conformation cannot perform their function
- Protein Modification
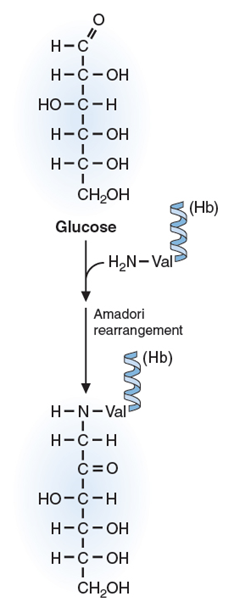
- Proteins can also be modified non-enzymatically, as hemoglobin is by glucose (increasing the HbA1c)
- The hemoglobin A1c (HbA1c) test measures the amount of blood sugar (glucose) attached to your hemoglobin. Hemoglobin is the part of your red blood cells that carries oxygen from your lungs to the rest of your body.
- The higher the concentration of glucose in the blood, the more likely “glycation” is to occur
- Glycation occurs when a sugar molecule stops circulation and binds to a protein like collagen thus destroying its function.
- Glycated hemoglobin causes an increase of highly reactive free radicals inside blood cells, altering the properties of their cell membranes.
- Advanced glycation end-products or “AGEs” result from “glycation”, these are pro-inflammatory molecules that are harmful to the cell
- Case Study 1
- A person with type 1 diabetes went to their healthcare provider for a check-up to monitor their treatment. Blood was drawn to check their glucose levels and monitor HbA1c. An HbA1c level of 8.5% (normal is 4-6%) was found.
- What does this mean?
- Why is HbA1c a good measure of blood glucose levels?
- Hemoglobin HbA1c
- Because the life of the red blood cell in the circulation is about 120 days, measuring the HbA1c gives a picture of what kind of conditions the red blood cells have been exposed to over the last few months
- The less well-controlled the blood glucose is and the higher the blood glucose, the more Hb will be “glycated”
- Case Study 2
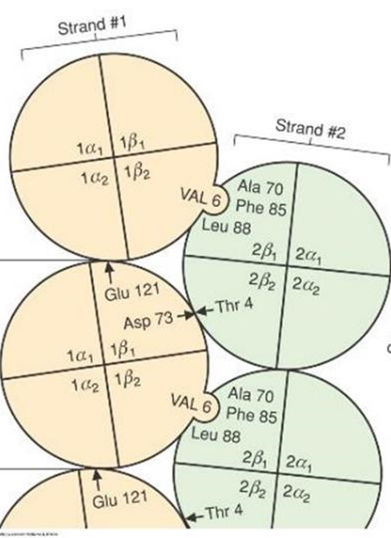
- The client with sickle cell disease again entered the hospital due to a sickle cell crisis. The are heterozygous for a mutation in their β-globin gene. They have that one amino acid changed from glutamate to valine. Due to this change, , hemoglobin can polymerize and sickle the cells, preventing oxygen delivery.
- What kind of amino acid change is a glutamate to valine?
Non conservative change
- What do you think this will do to the protein?
It will connect to other proteins in reach for hydrophobic interactions. Hence, allowing formation of long strands of hemoglobin.
- Consequences of Non Conservative change
- Because glutamate, with a negatively charged group has been replaced by the hydrophobic valine, it can interact with a hydrophobic pocket on another hemoglobin
- This allows the formation of long strands or polymers of hemoglobin
- This causes the red blood cell to sickle and prevents it from doing its job of delivering oxygen to tissues