mass spec
Sample is vaporised (g)
Carried out under a vacuum
Electron bombardment / electrospray ionisation
An electron gun fires high energy electrons at the sample, knocks an electron off leaving an X + (g)
X(g) --> X+ (g) + e-
Sample is dissolved in a volatile solvent ( vaporises easily). Injected through a hypodermic needle to give a fine mist ( aerosol)
Tip of needle is attached to a high voltage power supply - as it sprayed, sample gains H+
X(g) + H+ --> XH+(g)
Acceleration area
+ve ions accelerates towards the negatively charged plate
They accelerate until they reach the same Ke
Ke = 1/2mv2 v = d/t
ION drift
Ions pass through the flight tube and are separated based on mass
Ion detector
When ions reach the detector plate they gain an e-
This causes a current to flow
current is directionally proportional to abundance
Spectrum
Computer generated graph
what are the two methods of ionisation that could be used in a mass spectrometer
a. give the equation to show the formation of an ion of metal X for each method
b. describe each process
compare the speed and kinetic energy of a lithium and potassium ion in TOF mass spectrometry.
how are ions detected in a mass spectrometer
how can you determine the number of ions hitting the detector at m/z value
which of these ions will reach the detector first? 63CU+ or 65Cu+
give the equation to show the relationship between distance, time and velocity. state all units
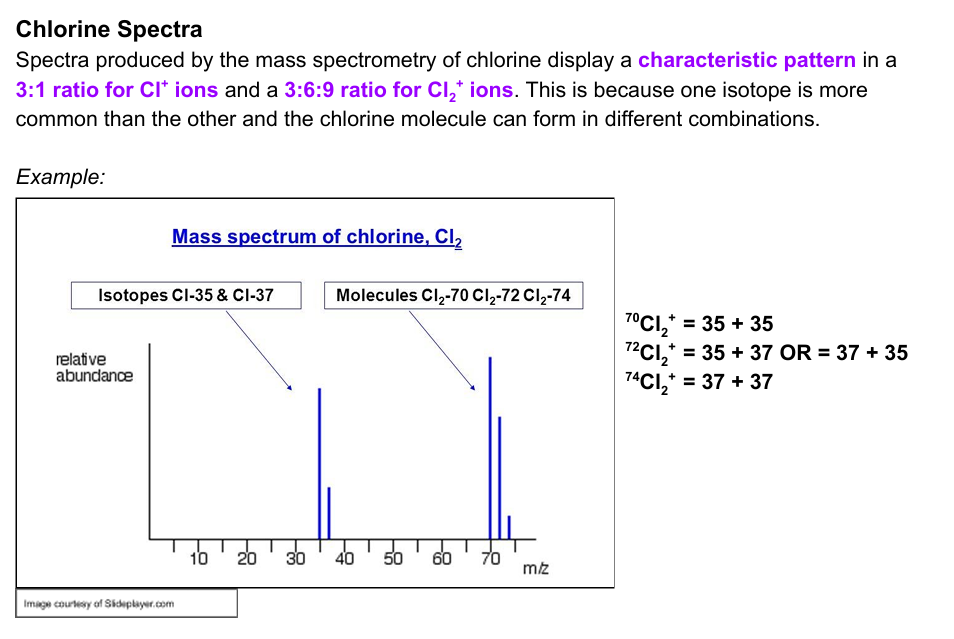
how many peaks would you expect in the spectra of a diatomic molecule that exists as two different isotopes. Explain you answer
calculate the mass in Kg of a Ni+ ion ( Mr = 58) using avagadro’s number (6.022×10²³)
a sample of copper contains two isotopes, 63-copper and 65-copper. all the ions were accelerated to have 1.130×10^-15 J of kinetic energy and travelled through a flight tube that was 0.700m long. 63Cu+ ions took 1.749 ×10^-5s. how long would 65Cu+ ions of mass 1.079×10-25 kg take to travel along the same flight tube.
how will the mass of a species ionised by electron bombardment differ to the mass of a species ionised by electrospray \