Cardiomypathy, Infectious, inflammatory Cardiac Disorders
Cardiomyopathy
Definition
Cardiomyopathy: Disease of the heart muscle associated with cardiac dysfunction.
Classified based on structural and functional abnormalities.

Classifications
Dilated Cardiomyopathy (DCM)
Most common form characterized by significant dilation of ventricles and systolic dysfunction.
Decreased ejection fraction and increased end-diastolic volume, leading to heart failure.
Hypertrophic Cardiomyopathy (HCM)
Characterized by increased heart muscle size, particularly along the septum.
Leading cause of sudden death in adolescents and young adults, especially athletes.
Restrictive (Constrictive) Cardiomyopathy (RCM)
Rigid ventricular walls resulting in impaired diastolic filling.
Symptoms similar to constrictive pericarditis.
Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia (ARVC/D)
Inherited form characterized by replacement of myocardium with scar and adipose tissue.
Unclassified Cardiomyopathy
Displays features of multiple types and caused by conditions like fibroelastosis and mitochondrial diseases.
Pathophysiology
Impaired cardiac output leads to decreased stroke volume, activating sympathetic nervous system and the renin-angiotensin-aldosterone response.
Resulting in increased systemic vascular resistance and fluid retention, increasing workload on the heart.
Clinical Implications
Sodium is the major electrolyte involved; often leads to fluid overload and heart failure.
Diagnosis often through echocardiogram (decreased ejection fraction).
Dilated Cardiomyopathy (DCM)
Characteristics
Distinguished by ventricular dilation without hypertrophy.
Leads to poor systolic function and high end-diastolic pressure, resulting in fluid overload and heart failure.
Causes
Over 75 possible conditions can cause DCM.
Idiopathic cases account for 20%-30% of nonischemic DCM cases.
Hypertrophic Cardiomyopathy (HCM)
Genetics and Risk
Autosomal dominant inheritance.
Increased muscle size leads to ischemia due to restricted blood flow.
Clinical Screening
Annual ECG, physical exam, and echocardiograms advised for asymptomatic patients with positive genetic testing.
Symptoms and Complications
Possible obstruction of left ventricular outflow tract (LVOT). Symptoms include dyspnea, syncope, and arrhythmias.
Management
Medical management through hydration, beta-blockers, or surgical options like myectomy or alcohol septal ablation.
Restrictive Cardiomyopathy (RCM)
Characteristics
Caused by rigid ventricular walls, leading to graduated increases in filling pressures and impaired diastolic function.
Symptoms similar to constrictive pericarditis, including dyspnea and chest pain.
Causes
May be either inherited or from systemic diseases. Diagnosis may require an endomyocardial biopsy.
Arrhythmogenic Right Ventricular Cardiomyopathy/Dysplasia (ARVC/D)
Clinical Presentation
Initially localized in the right ventricle; can progress to the left ventricle.
Symptoms may include palpitations, syncope, sudden cardiac death.
Diagnosis and Management
Diagnosis through ECG, echo, cardiac MRI, and family history. Genetic counseling recommended.
Unclassified Cardiomyopathies
Characteristics are mixed from other types and relate to various underlying conditions.
Diagnosis of Cardiomyopathy
Procedures
Effective diagnosis involves patient history, echocardiography, and ruling out other heart conditions.
ECG findings may show arrhythmias and hypertrophy indicators.
Management
Management includes identifying causes, correcting heart failure, and potentially implanted devices for arrhythmias.
Surgical Management
Heart Transplantation
Considered when heart failure progresses beyond medical treatment.
Challenges with donor availability and managing rejection versus infection.
Ventricular Assist Devices (VAD)
Used for patients awaiting transplant or with advanced heart failure.
Increased use in destination therapy for patients ineligible for transplant.
Total Artificial Hearts
Designed to replace both ventricles; complications include bleeding and infection.
Infectious Diseases of the Heart
Types of Infections
Infective Endocarditis
Infection of heart valve endothelium, diagnosed by blood cultures and echocardiography. Symptoms include fever and heart murmur.
Myocarditis
Inflammation of myocardium; can result in dilation and heart failure. Symptoms may include flulike symptoms, fatigue, and dyspnea.
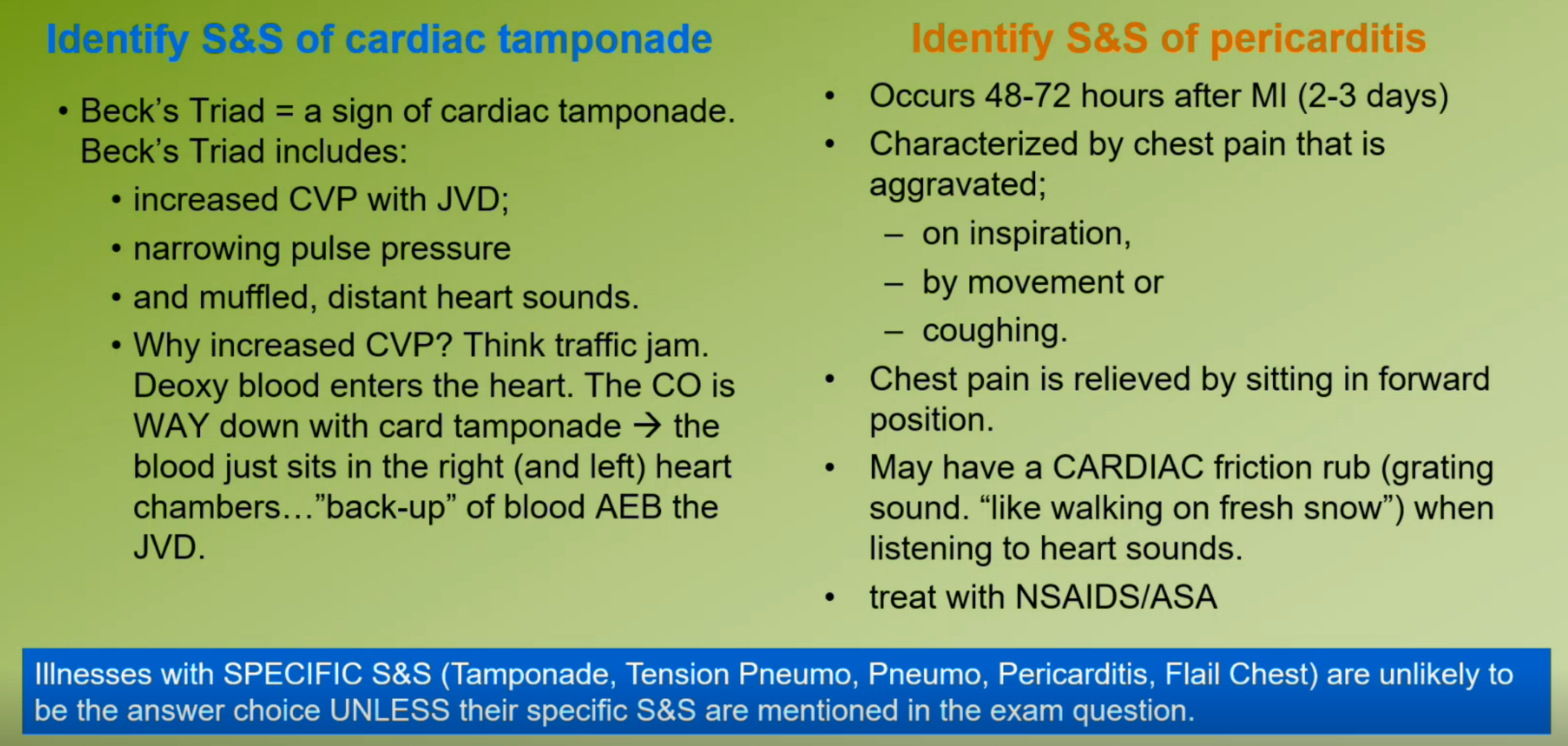
Pericarditis
Inflammation of the pericardium, symptoms include chest pain and can lead to effusion or cardiac tamponade.
Diagnosis and Management
Diagnosis often based on symptoms and echocardiography. Treatment includes IV antibiotics and may require surgical intervention for severe cases.
