2. Reactions of phenols
(c) Reactions of phenols
Following reactions are shown by phenols only
1. Electrophilic aromatic substitution
In phenols, the reactions that take place on the aromatic ring are electrophilic substitution reactions
The –OH group attached to the benzene ring activates it towards electrophilic substitution.
Also, it directs the incoming group to ortho and para positions in the ring as these positions become electron rich due to the resonance effect caused by –OH group.
The resonance structures are shown under acidity of phenols.
Common electrophilic aromatic substitution reactions taking place in phenol are as follows:
(i) Nitration: With dilute nitric acid at low temperature (298 K), phenol yields a mixture of ortho and para nitrophenols.
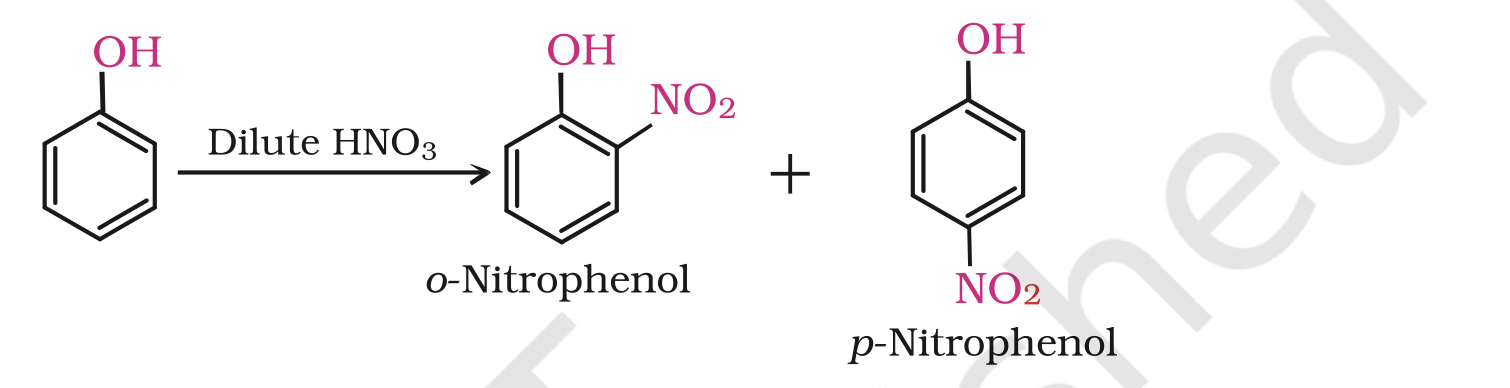
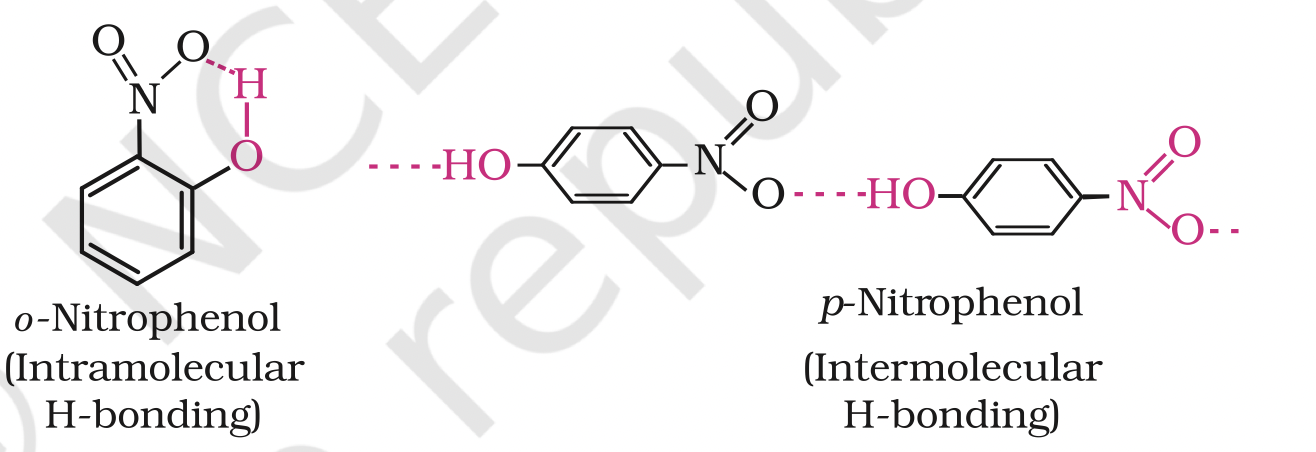
The ortho and para isomers can be separated by steam distillation. o-Nitrophenol is steam volatile due to intramolecular hydrogen bonding while p-nitrophenol is less volatile due to intermolecular hydrogen bonding which causes the association of molecules.
 Now a days picric acid is prepared by treating phenol first with concentrated sulphuric acid which converts it to phenol-2,4-disulphonic acid, and then with concentrated nitric acid to get 2,4,6-trinitrophenol. Can you write the equations of the reactions involved?
Now a days picric acid is prepared by treating phenol first with concentrated sulphuric acid which converts it to phenol-2,4-disulphonic acid, and then with concentrated nitric acid to get 2,4,6-trinitrophenol. Can you write the equations of the reactions involved?Phenol is first treated with concentrated sulphuric acid to form phenol-2,4-disulphonic acid:
C6H5OH + 2H2SO4 → C6H3(OH)(SO3H)2 + 2H2O
The phenol-2,4-disulphonic acid is then treated with concentrated nitric acid to form 2,4,6-trinitrophenol (picric acid):
C6H3(OH)(SO3H)2 + 3HNO3 → C6H2(NO2)3OH + 2H2SO4 + H2O(ii) Halogenation: On treating phenol with bromine, different reaction products are formed under different experimental conditions.
(a) When the reaction is carried out in solvents of low polarity such as CHCl3 or CS2 and at low temperature, monobromophenols are formed.
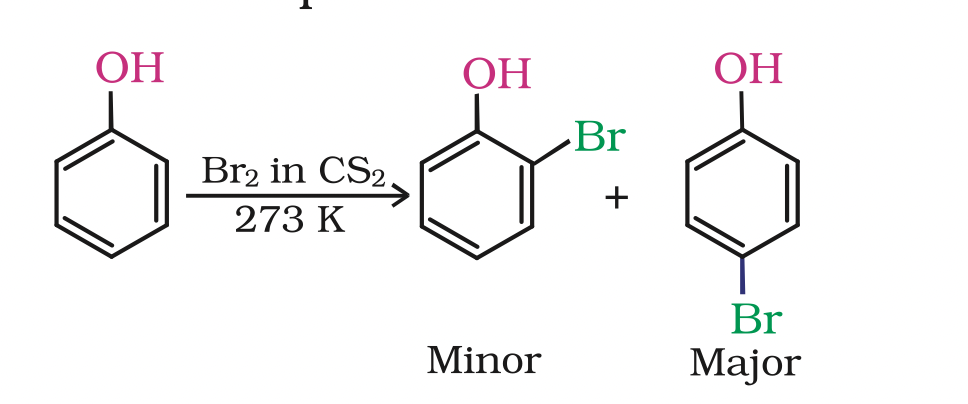 The usual halogenation of benzene takes place in the presence of a Lewis acid, such as FeBr3
The usual halogenation of benzene takes place in the presence of a Lewis acid, such as FeBr3 which polarises the halogen molecule.
In case of phenol, the polarisation of bromine molecule takes place even in the absence of Lewis acid. It is due to the highly activating effect of –OH group attached to the benzene ring.
(b) When phenol is treated with bromine water, 2,4,6-tribromophenol is formed as white precipitate.
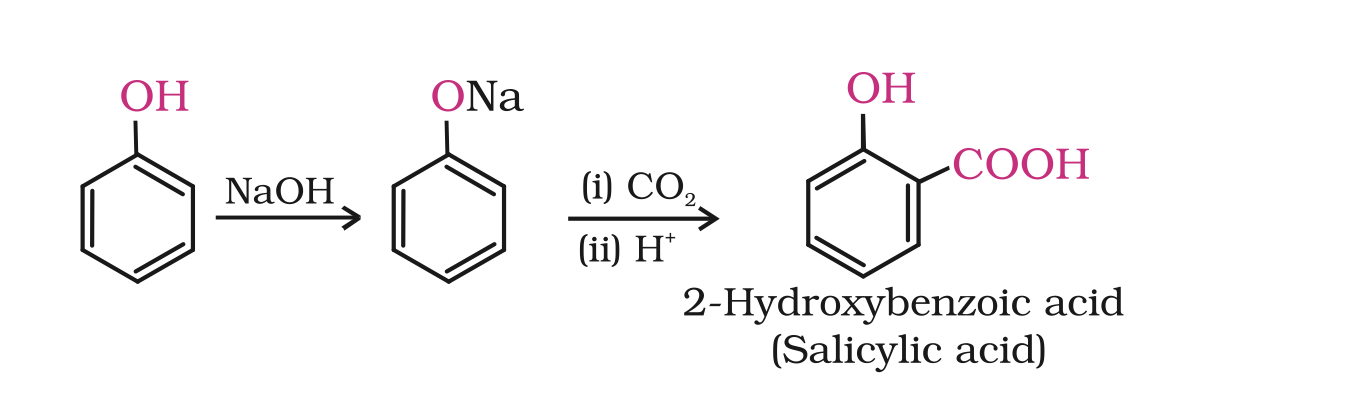
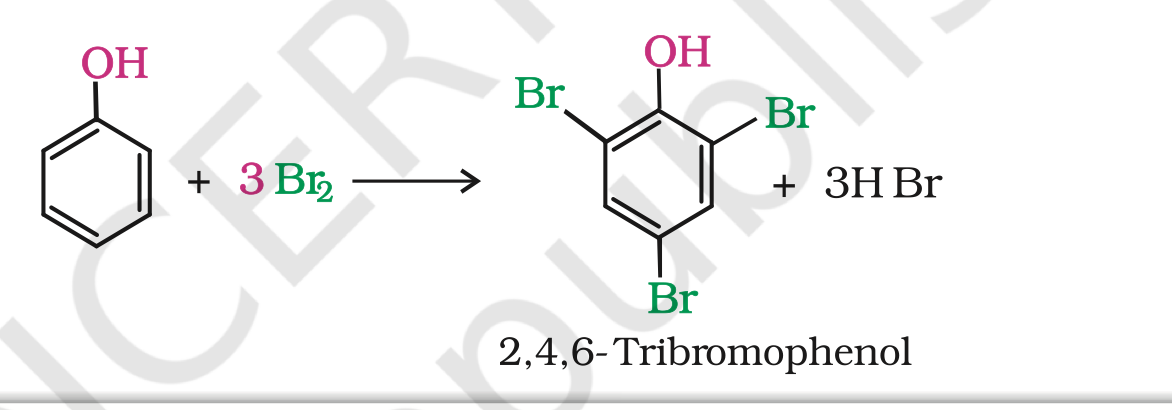 2. Kolbe’s reaction
2. Kolbe’s reactionPhenoxide ion generated by treating phenol with sodium hydroxide is even more reactive than phenol towards electrophilic aromatic substitution.
Hence, it undergoes electrophilic substitution with carbon dioxide, a weak electrophile.
Ortho hydroxybenzoic acid is formed as the main reaction product.
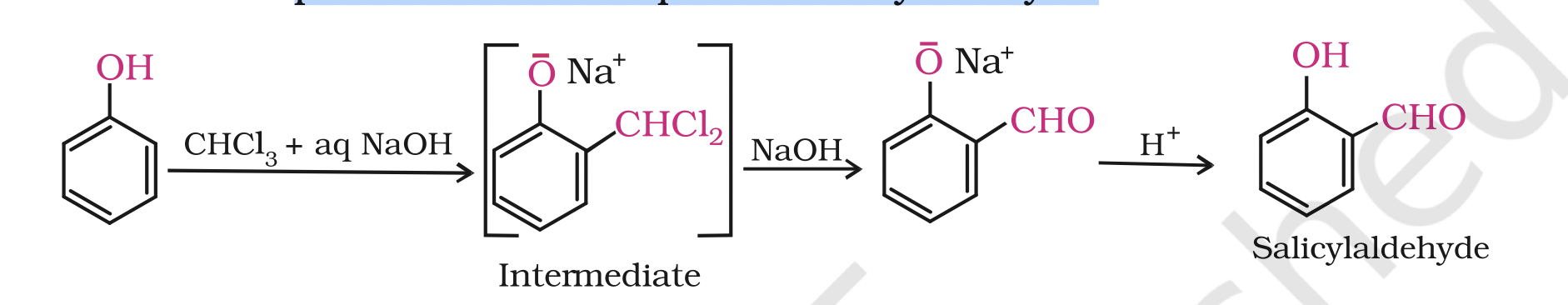
 3. Reimer-Tiemann reaction
3. Reimer-Tiemann reactionOn treating phenol with chloroform in the presence of sodium hydroxide, a –CHO group is introduced at ortho position of benzene ring.
This reaction is known as Reimer - Tiemann reaction.
The intermediate substituted benzal chloride is hydrolysed in the presence of alkali to produce salicylaldehyde.
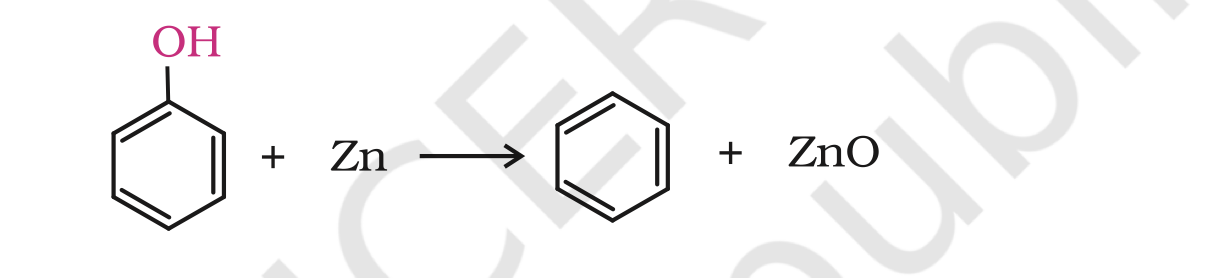
 4. Reaction of phenol with zinc dust
4. Reaction of phenol with zinc dust
Phenol is converted to benzene on heating with zinc dust.
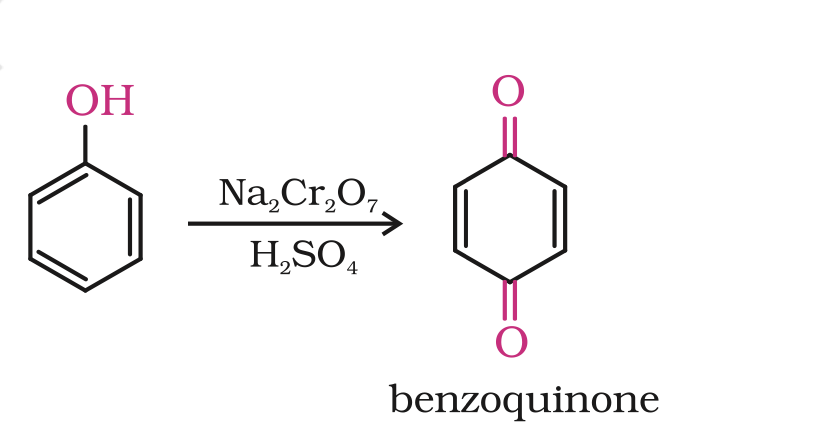
 Oxidation
OxidationOxidation of phenol with chromic acid produces a conjugated diketone known as benzoquinone.
In the presence of air, phenols are slowly oxidised to dark coloured mixtures containing quinones.