CHEMISTRY: ATOMS, COMPOUNDS, IONS (FORMULA AND SYMBOLS)
Atoms, Compounds, and Ions (Formulas and symbols)
How do you know what elements form the compound and in what proportions?
Atoms and compounds are electrically neutral (no net charge).
Atoms and compounds contain an equal amount of positively charged protons and negative charged electrons, which produces a neutral atom.
Ions are not neutral
Ions can be either positive or negatively charged.
Its charge depends on the amount of protons and electrons.
protons > electrons = positive charge
protons < electrons = negative charge
Ionic Charge
The charge of an Ion is indicated by a superscript following the symbol of the ion.
E.g.
A sodium ion of 1+ is written as Na+ (the 1 is omitted when an ion has a charge of 1+ or 1-)
Polyatomic ion: a group of atoms covalently bonded together
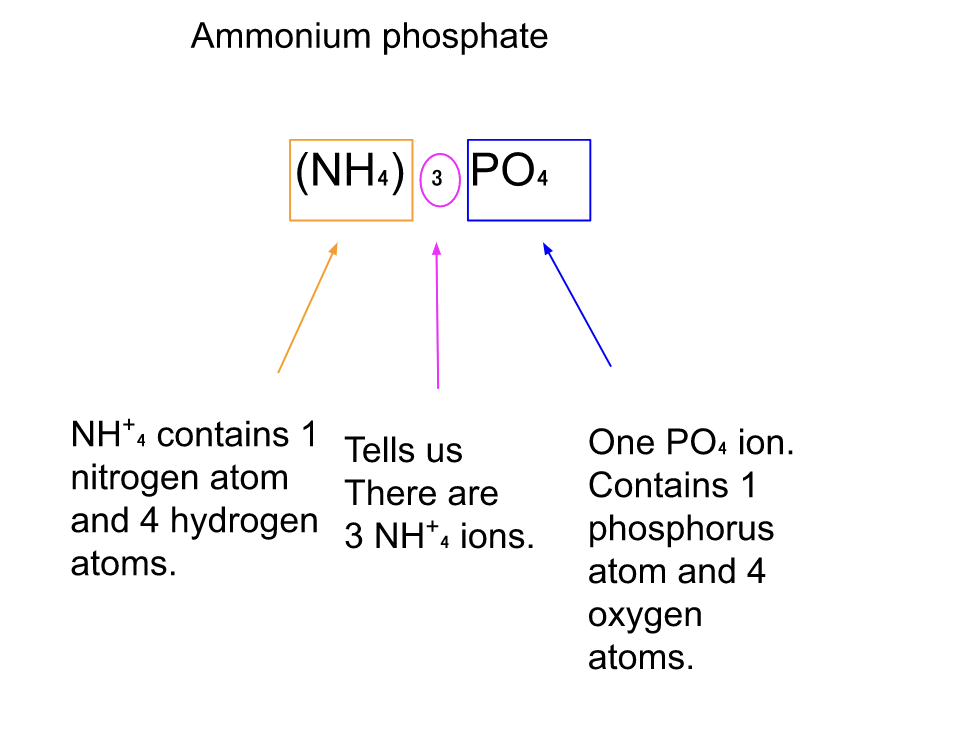
Ca(OH)₂ calcium hydroxide (NH₄)₃PO₄ ammonium phosphate
In the above are some examples of formulas that contain parentheses.
Parentheses are used to enclose polyatomic ions when there is more than one of the ions in a unit of a compound.
The Subscript after the parentheses tells us how many ions are present in the compound.
Subscript after elements simply refers to the number of atoms of each element.
E.g.
Coefficients
Sometimes there are coefficients written in front of a formula. The coefficient tells us how many units of the formula are present, and it applies to the entire formula.
E.g. 2H₂O means that there are 2 molecules of water (H₂O = water).
The two molecules of water both contain 2 hydrogen atoms and 1 oxygen atom.
In total the formula consists of 4 hydrogen atoms and 2 oxygen atoms.
To determine the number of atoms present, We multiplied the coefficient by the rest of the formula. 2 x H₂O
Try it yourself:
How many atoms are in 3Ca(NO₃)₂ ?
*Answer at the end
Hydrates
Compounds of hydrates are formed when crystal lattices from the cells form due to evaporated water in Ionic Solutions.
These crystals have a definite number of water molecules for each unit of a compound. The anhydrous (not hydrated) compound can be obtained by heating the crystals to drive off the water.
In a chemical reaction the water in hydrate does not react.
Answer: 3 calcium atoms, 6 nitrogen atoms, and 18 oxygen atoms.
Let’s start by figuring out how many atoms are in (NO₃)₂
NO₃ has 1 nitrogen atom and 3 oxygen atoms. Since we have parentheses, this tells us that there is more than one NO₃ ion. Based off the subscript 2 we know there are two NO₃ ions.
By multiplying 2 x NO₃ , we get a total of 2 nitrogen atoms and 6 oxygen atoms in (NO₃)₂
Now, let's look at the whole formula
3Ca(NO₃)₂
Since there is a Coefficient of 3, it should be multiplied by the rest of the formula.
3 x Ca = 3 Calcium atoms
3 x (NO₃)₂ = 6 nitrogen and 18 oxygen atoms
Therefore our answer is 3 calcium atoms, 6 nitrogen atoms, and 18 oxygen atoms.