Study Guide #4
Immunological Assays Study Guide
Learning Outcomes
Students will be able to ...
Explain how monoclonal antibodies are produced (mice).
Explain how antibodies are used in diagnostic kits and assays.
Identify the appropriate immunological assay appropriate for a given situation.
Important Terms:
Affinity: the strength of the interaction between the antigen-binding site on an antibody and the epitope on an antigen
Agglutination: a reaction in which particles suspended in a liquid collect into clumps and which occurs especially as a serological response to a specific antibody
Avidity: total strength required for the interaction between a multivalent antibody and multiple antigenic epitopes
Biotin: promoted for hair, skin, nail health
Enzyme-linked immunosorbent assay (ELISA): test to detect and quantify antibodies in the blood
Flow cytometer (FCM): a technology that provides rapid multi-parametric analysis of single cells in solution
Fluorescence-activated cell sorter (FACS): a specialized type of flow cytometry
Hybridoma: method for producing large numbers of identical antibodies
Monoclonal antibody: proteins made in a laboratory that act like antibodies in the body
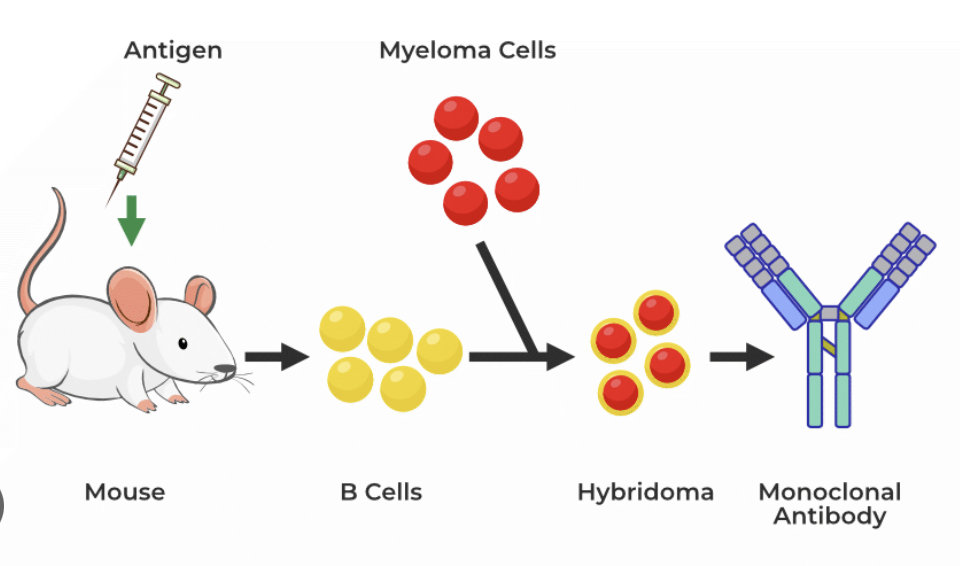
Myeloma cells: abnormal plasma cells that build up in the bone marrow and form tumors in many bones of the body
PAGE: high resolution technique commonly used for separating the mixture of proteins according to their molecular weight (separates big and small proteins, larger run slow, smaller run fast)
Polyclonal antibody: a complex mixture of several antibodies that are usually produced by different B-cell clones of an animal
Sodium dodecyl sulfate: detergent agent that breaks disulphide bonds of proteins disrupting the tertiary structure of proteins along with some reducing agent (allows a size separation)
Streptavidin: tetrameric protein commonly used in biotechnology as a stable linker due to its strong affinity for biotin molecules
Western blotting: a laboratory technique used to detect a specific protein in a blood or tissue (uses SDS, then transfer onto polymer that immobilizes proteins)
Important methods (helpful videos demonstrating the methods and explaining it):
Enzyme-linked immunosorbent assay (ELISA):
-Should be used: liquid (or liquified [SDS/boiling])
-Characteristic: Simple, sensitive, very quantitative
Flow cytometer (FCM):
-Should be used: cells in solution such as blood (tissues hard to analyze)
-Characteristic: very quantitative, limited information if simple system
Fluorescence-activated cell sorter (FACS):
-Should be used: cells in solution
Antibodies can have a fluoresent protein already attached
-Characteristixc: complicated system, very quantitative
PAGE:
Western blotting:
-Should be used: tissues or complicated samples
-Characteristic: complicated, very sensitive, but less quantitative
Microarray:
Hybridoma technology:
Concepts:
Why is there a problem with using immunoassays on patients taking biotin
supplements:
-If a patient is taking biotin, it can elevate concentrations in serum or plasma, which then can falsely increase analyte concentration when using competitive immunoassays. In contrast, biotin can show a negative interference if sandwiched ELISA is used.
- In sandwich ELISA, biotin can cause a problem with the monoclonal anti-body coated well in sandwich because it can displace what the antibody had affinity to.
Discuss the problems associated with the use of animal antisera:
-limited supply; each animal has only so much supply of antiserum and to get a big quantity you have to euthanize lots of animals.
Compare and contrast the advantages and limitations of pooled human Ig, and
monoclonal antibodies:
advantages for monoclonal antibodies: produced in large amounts outside of the immune system, less expensive
disadvantages for monoclonal antibodies: low expression of target antigens and immune responses to the antibodies, physical barriers preventing antibody binding
advantages of pooled human Ig: ensures the diversity of antibody
disadvantages of monoclonal antibodies: can cause really severe adverses
Recognize the components of a flow cytometry (FCM)
-fluidics: responsible for transporting samples from the sample tube to the flow cell, past the laser, sorted and/or discarded
-optics: induce excitation light sources, lense and optical filters used to collect and move wavelengths of light around the instrument and detection system that generates the photocurrent
-electronics: flow cytometer instrumentation
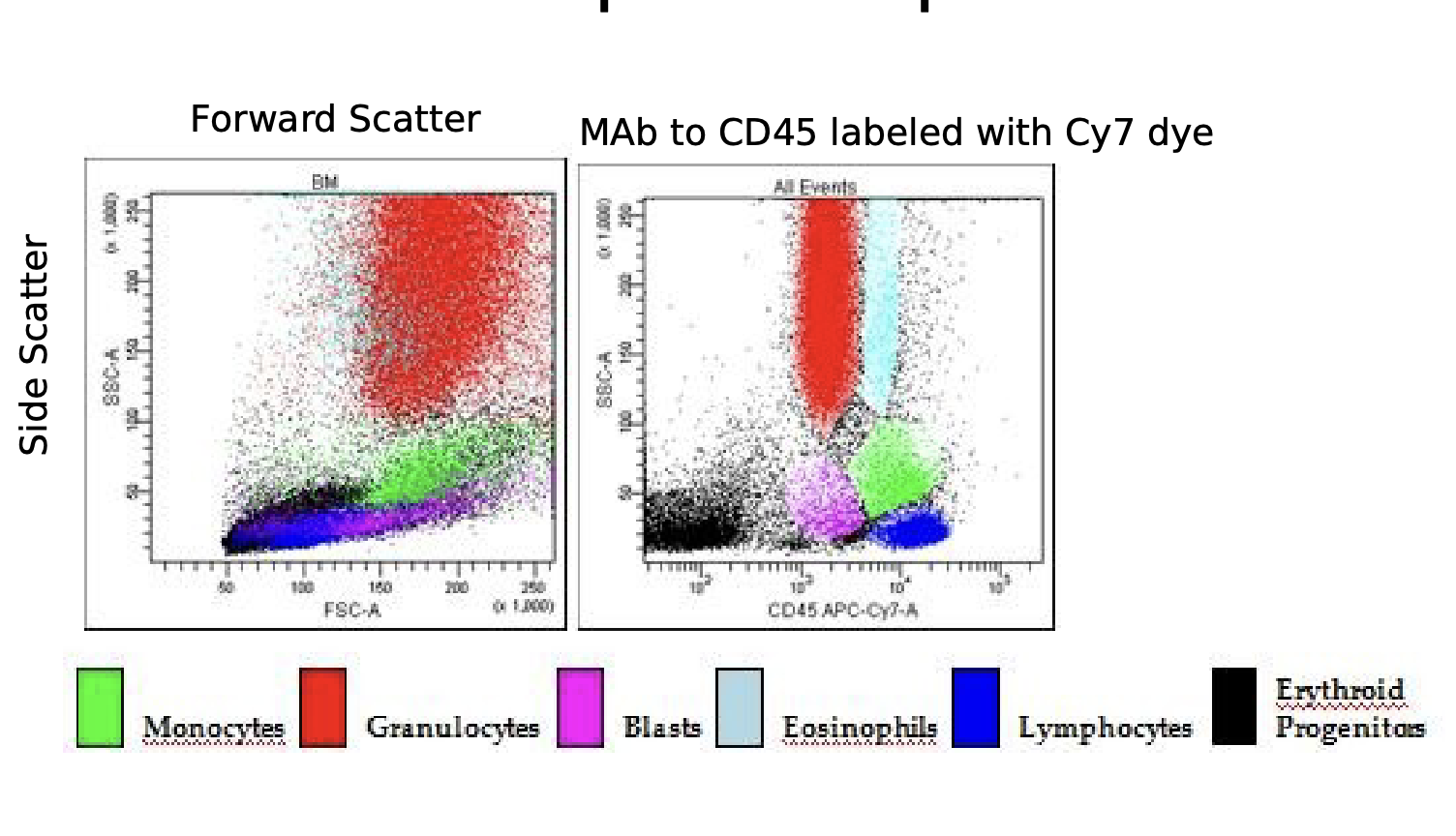
Compare and contrast forward and side scatter measurements
- Forward Scatter: one detector measures scatter along the path of the laser (size)
monocytes
-Side Scatter: the other detector measures scatter at a ninety degree angle (granularity)
Differentiate between absorbance and emission spectra:
-Absorbance spectra: atoms absorb energy
-Emission spectra: radiation emitted by excited atoms
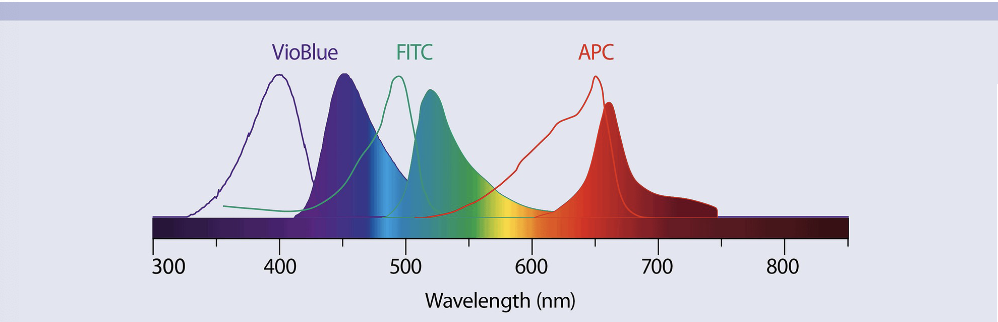
Identify common fluorochromes used in lymphocyte enumeration
-VioBlue (Purple), FITC (Blue/Green), and APC (Red/Orange) ____ don’t memorize the names just know that we can put these dyes on monoclonal antibodies and we can color the cells, we can tune our emission waves
Understand the concept of dual labeling and four-quadrant analyses
(I will update this next class, I don’t understand the concept)
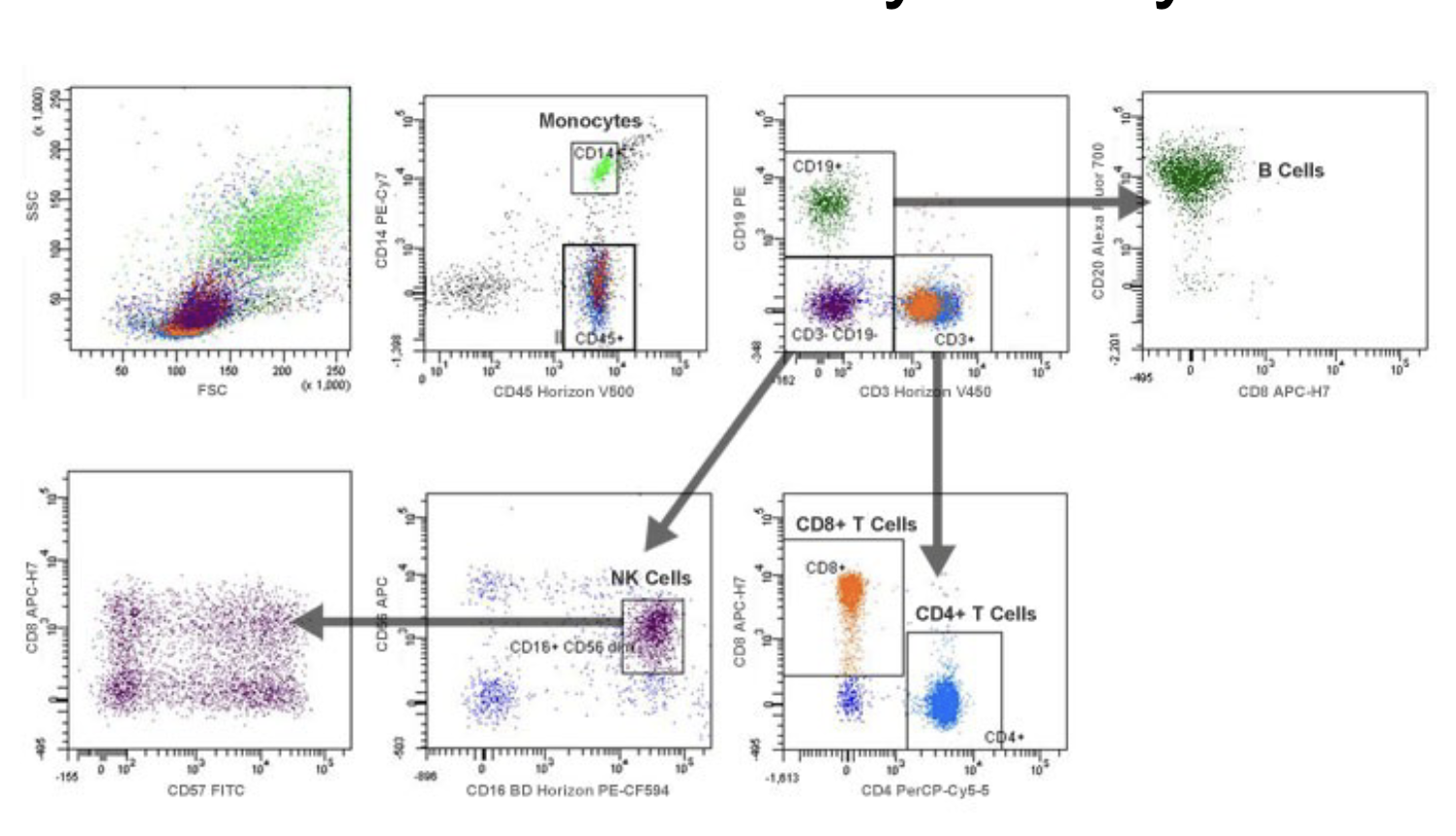
Compare and contrast the components of a flow cytometer and a fluorescence-
activated cell sorter (FACS)
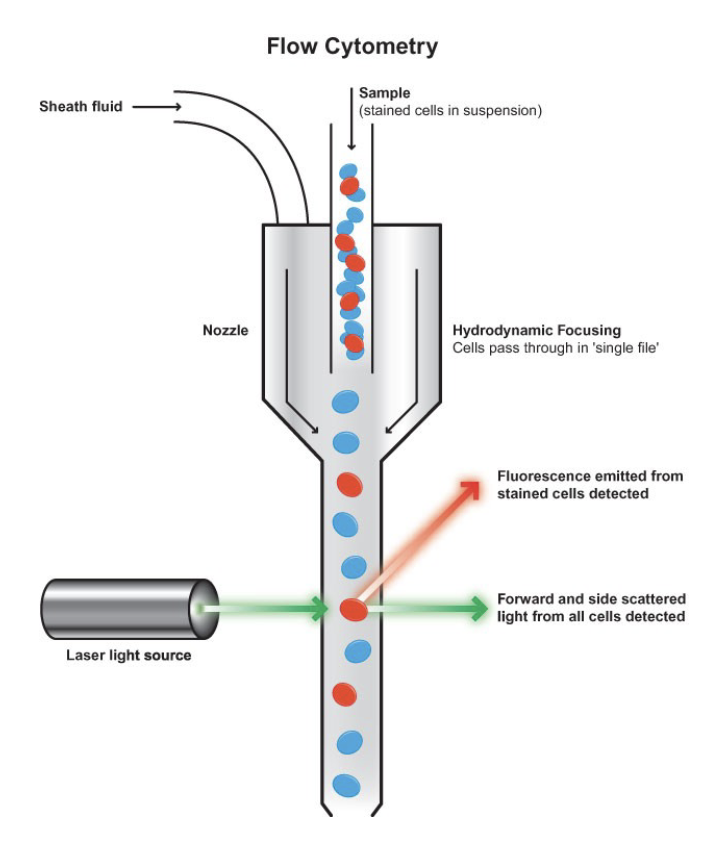
VS
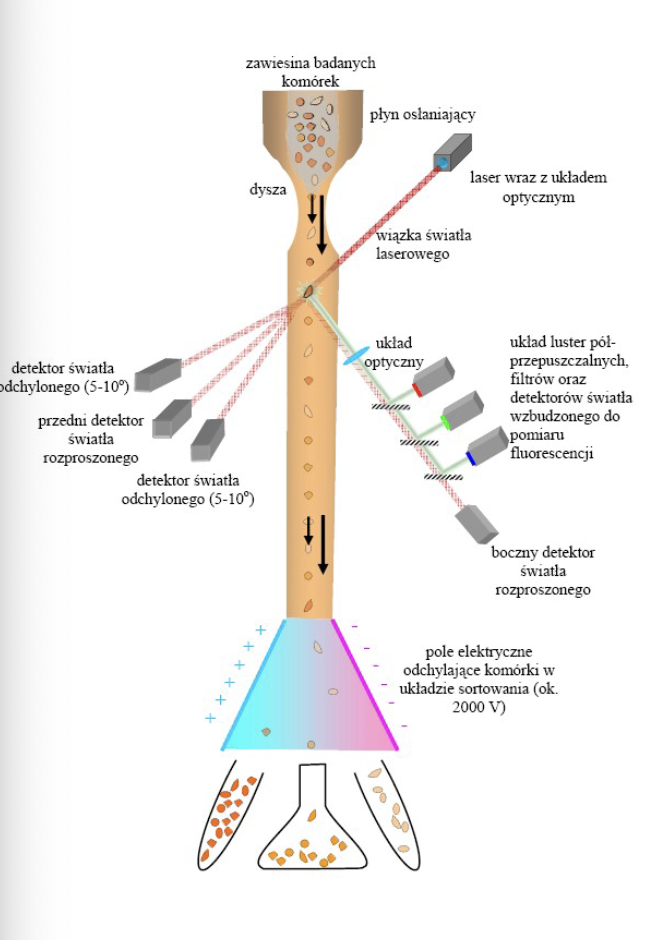
remembers what were the markers
Explain the lateral flow technology used in home pregnancy tests:
- Measuring human chorionic gonadotropin hormone (pregnant women make it very early on):
-Sample (urine) flows from left to right, first encounters antibodies to the analyte and the antibodies react to change colors (conjugated), sandwiched antibodies where test line is (this is where a positive result will be seen), control line has anti-antibodies (has to be a sample on the control line)
-Sample that is swirled into reagent (this is for COVID rapid test, works the same just with a different type of sample)
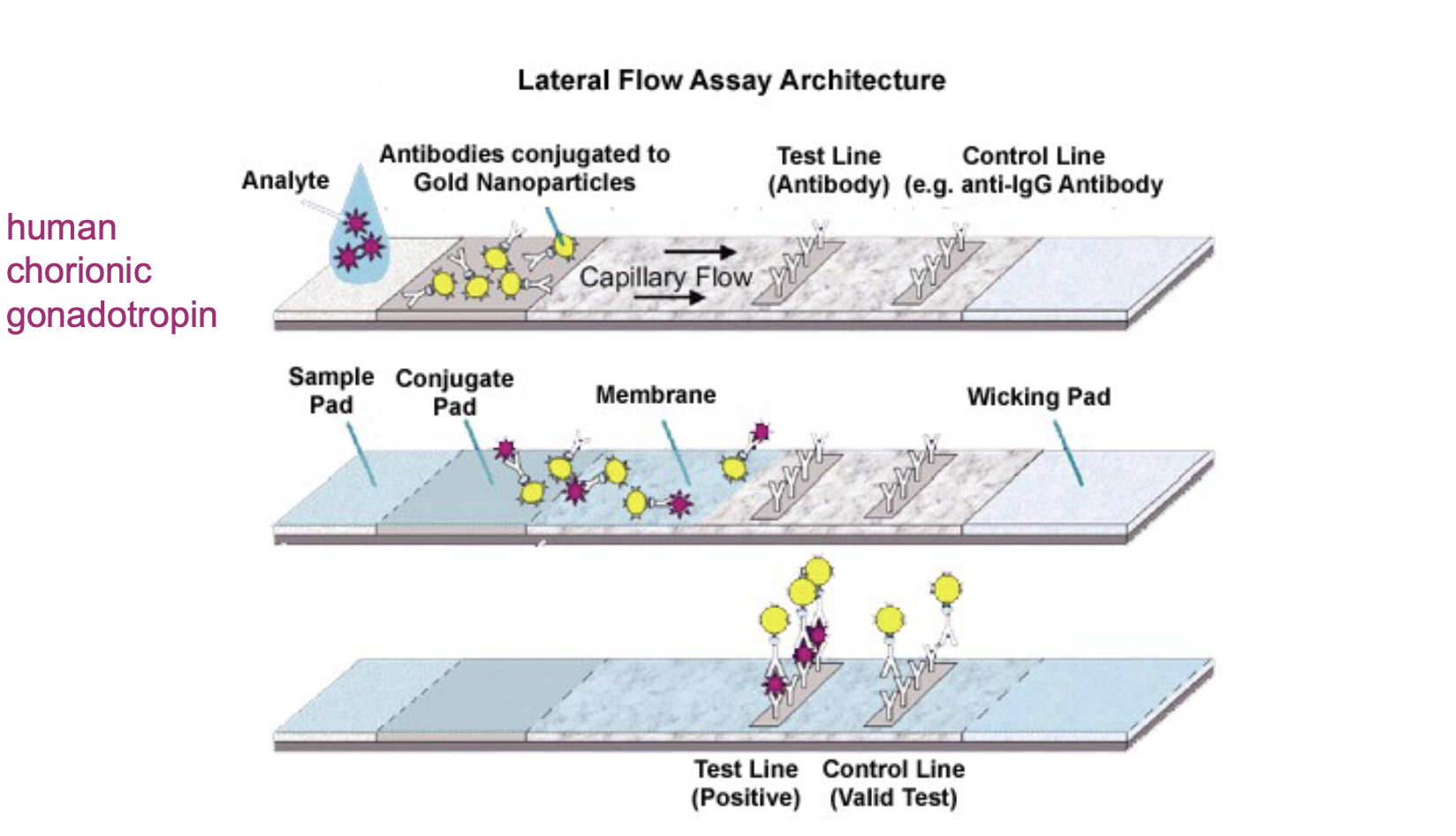
-What could go wrong? It could have gotten too hot and “cooked” the test
Compare and contrast the direct, indirect, and sandwich enzyme-linked
immunosorbent assay (ELISA) formats
-Designed to give a quantitative measurement.
-Indirect ELIZA: enzyme that is attached to the antibody. start out with antigen bound to polystyrene (highly charged plate), wash, have to coat the rest of not-binded sites, wash, enzyme-linked antibody binds to specific antibody, wash, substrate is added and converted by enzyme into colored product (the rate of color formation is proportional to the amount of specific antibody)
Video:
Explain Western blotting ( WATCH THE VIDEO)
-measures proteins
Recognize the function of sodium dodecyl sulfate in Western blotting
-imparts a relative negative charge proportional to their molecular weight, allowing separation of the protein by size only
Explain polyacrylamide gel electrophoresis(WATCH VIDEO FROM ABOVE)
Explain how monoclonal antibodies are created from mice
-Video: