Chemistry-Atoms
Atoms are the basic units of matter and consist of three primary subatomic particles: protons, neutrons, and electrons.
matter and atoms
matter
a substance that has volume (occupies space) and has mass
All matter is made up of atoms, which bond together to produce different substances
Matter and energy are not the same thing
Everything is either matter or energy
Energy does not have any atoms
Energy and matter can change into each other and vice versa
The first element to be found in the world
atoms
atom: building block of matter
1802: The first atomic theory of matter was presented by John Dalton. Dalton proposed that all matter is made up of tiny spherical particles, which are indivisible and indestructible
We now know it is INCORRECT and atoms are made of smaller subatomic particles (protons, neutrons and electrons)
Pure substances VS Mixtures
Matter can be split into two groups: pure substances and mixtures
Mixtures
Mixtures: consist of 2 or more types of particles that are not chemically combined. Can be made of elements, compounds or both
air: oxygen gas (O2), Carbon dioxide gas (CO2), Nitrogen gas (N2)
Oil-water mixture
Mixtures can be separated by physical means (e.g. filtration)
Pure Substances
Pure substances: A pure substance is a substance made up of only one type of particle throughout. This means it has fixed composition and consistent properties. This can either be one single element or one single compound, but every sample of this substance that you examine must contain the same thing with a fixed, definite set of properties
pure element: copper metal (cu)
Pure compound: carbon dioxide gas (CO2)
It cannot be separated by physical means (e.g. filtration)
Elements
Elements: Made of just one type of atom
It could be monatomic (i.e exist as individual atoms) or could also form molecules
molecules: 2 or more atoms that are held together by chemical bonds
elements cannot be separated into simpler substances by physical or chemical means
Compound
Compound: Different types of atoms could combine to form new substances
To determine whether a pure substance is an element or a compound, you must decide if the substance can be broken down into simpler substances
Element | Compound |
|
|
Molecules: 2 or more atoms that are covalently bonded
MOLECULES MUST BE COVALENTLY BONDED
All molecules are compounds, but not all compounds are molecules
Compounds are ionically bonded
Atomic structure
An atom is made up of three types of subatomic particles:
protons (positively charged)
Neutrons (neutral)
Electrons (negatively charged)
In chemistry, the word particle is a general term that refers to a small unit of matter
Depending on the context, “particle“ could mean an atom, a molecule, an ion, or something else.
Atomic structures
Protons and neutrons have relatively equal mass and are huge in comparison to electrons. This is why the nucleus is so dense
Proton and neutron contribute nearly all the mass of the atom
Particle | Symbol | Charge | size relative to a proton | mass (kg) |
proton | p | +1 | 1 | 1.673×10-27 |
nucleus | n | 1 | 1 | 1.675×10-27 |
Electron | e | -1 | 1/1800 | 9.109×10-31 |
Protons and neutrons are relatively equally massive and are huge in comparison to electrons. This is why the nucleus is dense
Proton and neutron contribute nearly all the mass of the atom
The Rutherford Experiment
Rutherford’s model proposed the following
Most of the mass of an atom and all of the positive charge must be located in a tiny central region called the nucleus
Most of the volume of an atom is space, occupied only by electrons
The electrons move in a circular orbit around the nucleus
The force of the attraction between the positive nucleus and the negative electrons is electrostatic.
Before Rutherford’s experiment, the atom was thought to be a spherical cloud filled with protons and electrons all over (think raisins caked, or plum pudding)
In 1911, Rutherford’s experiment proved that the atom has a tiny but heavy nucleus and that most of the volume of an atom is space occupied by electrons
Bohr model
In 1913, Niels Bohr developed a new model of the hydrogen atom that explained emission spectra. The Bohr model proposed the following
electrons revolve around the nucleus in fixed, circular orbits
The electron’s orbits correspond to specific energy levels in the atom
Electrons can only occupy fixed energy levels and
Scientists quickly extended Bohr’s model of the hydrogen atom to other atoms
They proposed that electrons were grouped in different energy levels, called electron shells
These electron shells are labelled with the number n = 1, 2, 4
The electron shells
The highest energy level found is n7
The highest energy level is found farther away from the nucleus because of the electrostatic attraction force
The higher up you go, the higher the electrostatic attraction; therefore, the lower the energy, the closer you get to the nucleus
If 2 negatively charged electrons share an electron shell, they have anti-clockwise spins, so they never meet or come in contact with one another
think 2 siblings circling each other
Different types of atoms
The type of atom that makes up each element is determined by the number of protons (atomic number) in the nucleus
atomic number: the number of protons in the nucleus of the atom
mass number: the total number of protons + neutrons in the nucleus
Atoms are electrically neutral; therefore number of electrons = the number of protons
The number of neutrons is calculated by: atomic mass - atomic number (number of electrons/protons)
The atomic number is usually the smaller number, and the atomic mass is usually larger and has a lot of decimals
Isotopes
all atoms that belong to the same element have the same number of protons in the nucleus and therefore the same atomic number,
Atoms that have the same number of protons (atomic number) but different numbers of neutrons (and therefore different mass numbers) are known as isotopes
Isotopes have identical chemical properties but different physical properties, such as mass and density. In particular, some isotopes are radioactive
In nature, different elements have different numbers of isotopes. Gold only has one isotope, whereas lead has four isotopes, and mercury has seven isotopes
Protons: determine elemental identity
Electrons: determine chemical reactivity(how easily (or not easily) it is for the atom to undergo a chemical reaction )
Neutrons: determine physical properties the physical properties of an element
Practice with Isotopes
Identify the most abundant form of carbon out of the 3
126 C - Carbon 12
136 C - Carbon 13
146 C - Carbon 14
Carbon 12 - it appears in the periodic table, meaning it is the most commonly found Isotope
Note that 12, 13, and 14 are the atomic masses of the atom
Electron configuration
Using the Bohr model, it is possible to determine the basic electronic configuration of atoms by applying the following rules
Each shell can only contain a maximum number of electrons
Lower energy shells are full before higher energy shells
electron shell n | maximum number of electrons |
1 | 2 |
2 | 8 |
3 | 18 |
4 | 32 |
n | 2n2 |
Steps to annotating an atom - Electron configuration
Determine the number of electrons in your element
Recall the maximum number of electrons each shell can hold
Place the number of electrons in the shells from the lowest energy to the highest energy. Do not exceed the maximum number of electrons allowed
Write the electronic configuration by listing the number of electrons in each shell separated by commas. (2, 8, 1) (for sodium)
THIS ONLY APPLIES TO THE FIRST 18 ELEMENTS (TILL ARGON)
Atoms
Atoms: Building block of matter
Ions
A positively or negatively charged atom or group of atoms
Remember that an atom has the same number of electrons and protons, which means that it is neutral
When atoms pick up an additional electron (s) or lose electrons (s), there is no longer a balance between the positive and negative charges
They lose electrons/protons to achieve a full valence shell (octet rule)
An exception to the octet rule would be the duet rule (applies to hydrogen & helium)
The duet rule states that there would be 2 electrons in the valence shell
The 2 main types of ions
monoatomic, e.g.
a. Na+, Cl-, Cu+2
Polyatomic
a. e.g. pos43-, OH-
Cations
a positively charged ion (an atom loses electrons)
Meg2+, Al3+
Magnesium, for example, has 2,8,2
It loses 2 electrons and becomes
Mg +2/2+
You would also put square brackets around the visual representation and put a 2+/+2 in the upper right corner
Anions
Negatively charged ion (atom gains electrons)
e.g. Cl-, O2-
Chlorine, for example, has 2, 8, 7
It gains electrons and becomes
Cl-
There is an implied one
Metals
Metals have loosely held outer shells (valence electrons)
They can lose these electrons to become cations, this is because the resulting ion has fewer electrons than protons
Nonmetals
Non-metals attract electrons
Transition metals
They can take on more than 1 charge
They have variable charges
Really big valence shells so they can lose or gain electrons easily
positive control: variable with known result (e.g. plant is exposed to sunlight)
negative control: absence of IV (e.g. plant exposed to no light)
No result (e.g. no change in plant height/mass)
Electron Transfer and Ionic Bonding
Recall classifying matter
atom: the smallest particle of matter
Element: a substance made from only one type of atom
Compound: A substance made from 2 or more different types of atoms
Molecules: A substance made from two or more atoms that have chemically combined
Ionic compound
An ionic compound typically forms when a metal reacts with a nonmetal
Ionic compound = metallic cation + non metalli anion
During a reaction, there is a transfer of electrons. Metals → Non-metal
Once this occurs, the oppositely charged ions join together in a lattice
Oppositely charged ions are attracted to one another, and this attraction would be called an “electrostatic attraction”
electrostatic attraction, AKA ionic bonds, AKA the attraction between oppositely charged ions
Model | Example | does not show |
chemical formula | NaCl |
|
Dot and cross diagram |
| |
2D diagram |
| |
3D diagrams |
|
They are brittle, as when a force is applied to the lattice, the ions with like charges align and actively repel each other, shattering the lattice
The ionic bond is very strong and brittle because rearranging ions causes repulsion between like charges
You are sliding the layers so the bond is still intact, but the layers slide and the negatives end up next to negatives, so they repel each other, and then the lattice breaks apart
They normally have very high mpts
Energy must be transferred to a substance to make it melt or boil
This energy overcomes the strong electrostatic forces of attraction, which act in all directions between the oppositely charged ions
Some forces are overcome during melting
All remaining forces are overcome during boiling
The more energy needed, the higher the melting point or boiling point
Since the electrostatic forces of attraction between oppositely charged ions are strong, their melting and boiling points are high
Solid sodium chloride needs a temperature of 801 degrees to melt it
Electron transfer diagrams
Electron transfer diagrams are used to show the path that electrons take when they are removed from a metal and added to a non-metal during ionic bonding
show the transfer of electrons from metals to non-metals
Steps to draw an electron transfer diagram
Draw an electron shell diagram of the neutral metal and non-metal
Add a ‘+‘ between them
Draw an arrow leading from each valence in the metal to the valence shell of the non-metal
Add an arrow towards the resulting ions
Draw an electron shell diagram of the resulting cation/s and anion/s
Write the chemical symbol of the metal and the non-metal in the centre of the electron shell diagram, taking care of the electron shell diagram, taking care to add charges to your ions.
Naming Simple Ionic Compounds
RULES:
Name the cation first (metal) before the anions (non-metals)
*If metal is a f metal is a transition metal, indicate the valency in numerals after the name
E.g. Fe(III), Ag(I), Gold (I)The name of the cation remains as is
E.g., Sodium ion, Na+ ion
The name of the non-metal anion is changed. Its suffix becomes ‘-ide’
Simple Ionic Compound Formulas
naming compounds
Transition metals can take on more than one charge; in this case, indicate the charge using Roman numerals, which are in brackets.
Start with the positive ion or cation (the metal)- ALWAYS- and then follow it with the negative ion or anion (the nonmetal).
Drop the word cation after the positive ion
Metallic bonds
consists of the attraction between positively charged metal ions and delocalised electrons, allowing for malleability and conductivity. (can only be cations)
They tend to lose their outer shell electrons easily and turn them into positively charged cations.
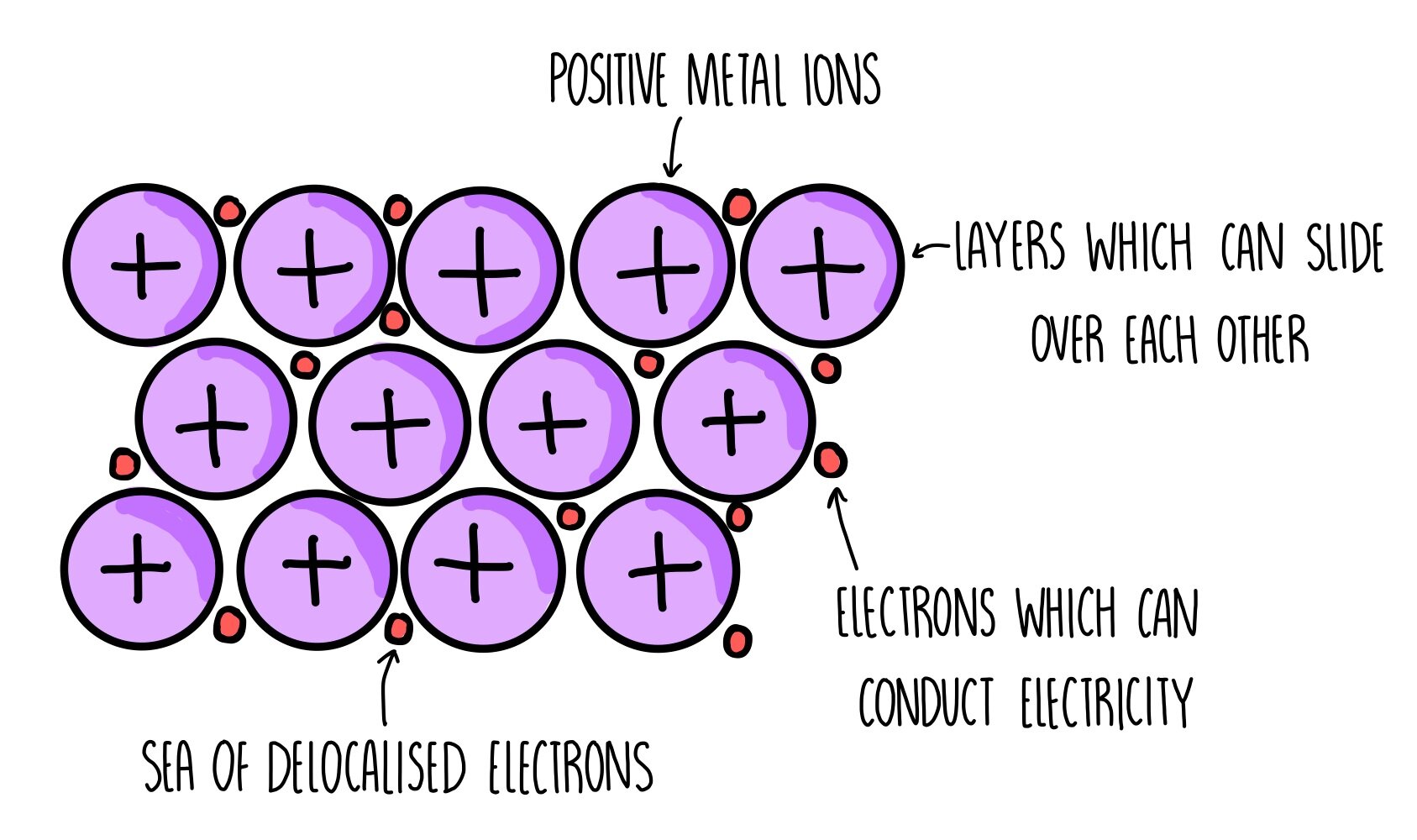
the electrostatic force of attraction between the positively charged metal cations and negatively charged valence electrons occur in all
Alloys
a mixture of two or more metals, combined by metallic bonding
they tend to harder than pure metals because pure metal atoms being the same and arranged in layers , as opposed to alloys that contain element with atoms of different sizes
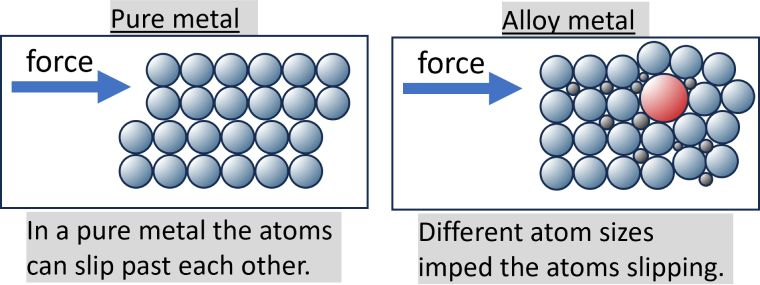
common alloys:
Steel: iron(metal)+ carbon(non-metal)
Bronze: copper(metal)+ tin(metal)
Brass: copper(metal)+ zink(metal)
Types of bonds
ionic | covalent | metallic | |
how are bonds formed | electrons are transferred between ions | electrons are shared between atoms | A “sea of delocalised electrons“ spreads over and between all atoms of the same element |
type of bond formed | solid crystals with repeating patters of positively and negatively charged ions | usually liquid or solid | solid metal |
involves | metal + non metal | non metal + nonmetal | Metal + metal |
conductivity | conducts electricity when dissolved in water | does not conduct electricity | very good conductor of electricity |
Dive deeper in ionic subsistences
ionic compounds are solid at room tempt
Ionic compounds are called ‘salts‘ and are solid at room temperature
ionic compounds have strong electrostatic forces of attraction between their positive and negative ions and form a lattice of ions
a high amount of energy is required to over come these forces
therefore, they exist as solids at room temperature
to shatter an ionic bond requires a ridiculous amount of energy
so inonic bonds are the strongest bonds
high melting points
high melting points in the ionic bonding model result from the strong electrostatic force by oppositely charged ions
to melt an ionic compounds, a large amount of energy is needed to break this strong bond and allow ions to move freely
as mentioned before, this means they are solid at room temperature
why are ionic compounds brittle
ionic compounds are brittle because, when external force is applied to them, the charged ions (cations + ions) shaift therefore align with ions
this causes them to repel each other, causing them to shatter
ionic compounds can be soluble in water
water can break apart the ionic lattice because the positive cations and negative anions are attracted to the partial charges within the water molecules
the kinetic energy of the water molecules breaks the electrostatics attractions inside the ionic lattice structure
this allows water to surround the individual ions furing dissolving
conduction of electricity in an aqueous solution
substances that conduct electricity have free moving, charged particles
when the charged ions are dissolved in water they can move to conduct electricity
if you are able to push particles easily through matter you can conduct electricity through it
they are conductive when in molten form
molten ionic compounds can conduct electricity because they have free mobile charged ions