cell membranes transpiration q&a
introduction to cell membrane and transport
spontaneous activity; lacks the traditional structure of lecture notes.
overview of active and passive transport mechanisms, with a primary focus on osmosis.
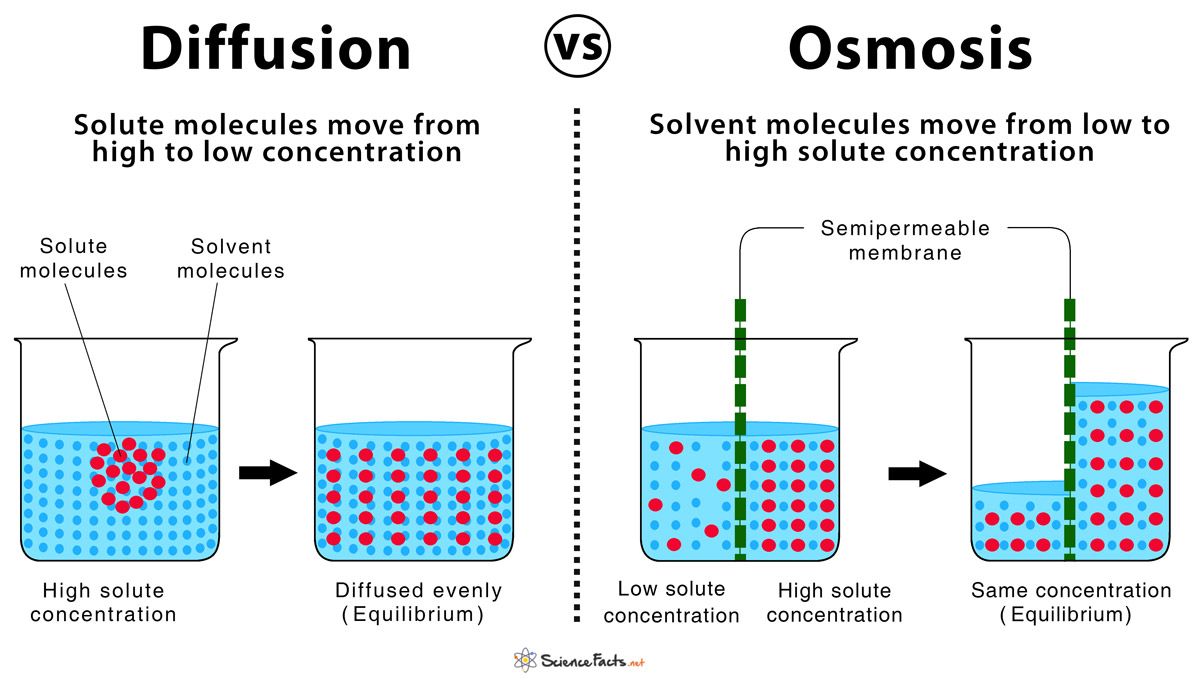
diffusion and osmosis overview
diffusion: movement of molecules from an area of higher concentration to an area of lower concentration (passive transport).
example: dye dropped in water, disperses due to diffusion.
relates to concepts like concentration gradient and brownian movement (random molecular motion).
osmosis: special type of diffusion that refers specifically to the movement of water.
water moves from a region with a higher concentration of water (less solute) to a region with a lower concentration of water (more solute).
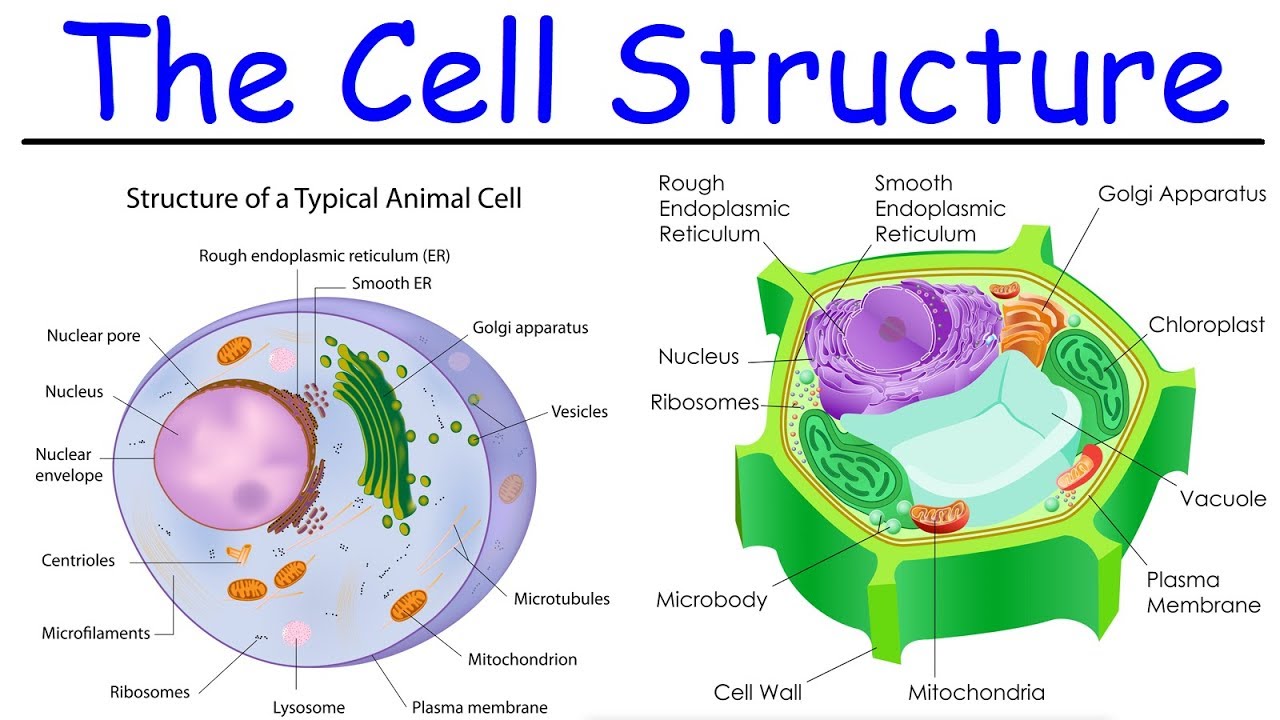
structure of the cell membrane
plasma membrane / cell membrane: a semi-permeable barrier that surrounds and protects the cell.
analogous to flexible plastic wrap; allows selective passage of molecules.
comprises a phospholipid bilayer featuring hydrophilic heads and hydrophobic tails.
function: regulates what enters and exits the cell, providing communication and signaling.
key functions of the plasma membrane
acts like a 'traffic cop' controlling the movement of substances.
small, nonpolar molecules pass freely, while larger molecules are typically blocked.
does not provide structural protection (unlike cell walls found in plant cells or bacteria).
roles of membrane proteins:
transport channels or gates for specific molecules.
role in immune recognition (e.g., id badge function to avoid immune attacks).
passive vs. active transport
passive transport: movement that does not require energy (atp) and occurs naturally along the concentration gradient.
includes the processes of diffusion and osmosis.
active transport: requires energy to move substances against their concentration gradient.
involves membrane proteins and atp. ex: sodium-potassium pump.

types of solutions
tonicity: measures the osmotic pressure gradient of two solutions separated by a semipermeable membrane. example: freshwater fish in freshwater (hypotonic) versus saltwater fish in saltwater (hypertonic)
hypotonic: lower solute concentration compared to the cell. causes cells to swell due to water influx (risk of lysis).
example: pure distilled water is hypotonic and could burst red blood cells.
isotonic: equal solute concentration inside and outside the cell. no net water movement; cells remain stable.
example: normal saline solution (0.9\% \text{ NaCl}).
hypertonic: higher solute concentration compared to the cell. causes cells to shrink due to water loss (risk of crenation).

example: saline solutions with a concentration above 0.9\% \text{ NaCl} .
experimentation with osmosis and diffusion
utilization of dialysis tubing as a model for studying osmosis and diffusion in a lab setting.
experiment: filling tubing with sugar solutions and placing in different beakers to observe effects.
hypotonic solution: tubing in distilled water should swell (water influx).
isotonic solution: keeping the same concentrations prevents net gain/loss of water.
hypertonic solution: tubing in a high sugar solution should shrink due to water being drawn out.
brownian motion and random molecular movement
brownian motion: the random movement of particles suspended in a fluid.

key concept for understanding diffusion and osmosis in both liquids and gases.
real-world applications and implications
understanding diffusion and osmosis is critical in various fields like medicine (e.g., dialysis treatment for kidney failure).
concept of homeostasis is significant; cells must maintain balance in their internal environment for proper functionality.
conclusion
importance of the plasma membrane in cellular transport and overall cell health is vital to biological systems.