Factors affecting enzyme activity
Temperature
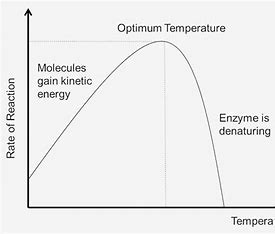
At low temperatures, the rate of an enzyme-catalysed reaction is low because the enzyme and substrate have less kinetic energy. Therefore, there are fewer, less frequent collisions between the substrate and active site and fewer ESC form, so less substrate is converted into product. As the temperature increases, the rate of reaction increases as more successful collisions occur between the substrate and active site to form more ESC, until the optimum temperature for the enzyme is reached when the rate of reaction reaches a maximum. As temperature increases above the optimum temperature the rate of reaction starts to decrease as the enzyme becomes denatured. The kinetic energy of the enzyme increases, so the enzyme vibrates too much breaking the weak bonds e.g. hydrogen bonds that maintain the tertiary structure of the enzyme. Therefore, the enzyme changes shape and the active site changes shape, so it is no longer complementary in shape to the substrate. The substrate can no longer bind to the active site to form an ESC. As a result, less substrate is converted into product so the rate of reaction decreases.
Extremophiles that live at very hot temperatures will have enzymes that can remain active at these temperatures. This is because their tertiary structure is maintained by more strong covalent disulfide bonds and less hydrogen bonds.
Temperature coefficient
The temperature coefficient (Q₁₀) refers to the increase in the rate of a process when the temperature is increased by 10°c.
Q₁₀ = Rate of reaction at (t + 10)°c / Rate of reaction at t°c.
For enzyme-controlled reactions between 0°c and 40°c, Q₁₀ is approximately 2, which means the rate of reaction doubles for every 10°c rise in temperature.
pH
pH is a measure of hydrogen ion/proton concentration. Low pH, acidic solutions have a high hydrogen ion concentration while high pH, alkaline solutions have a low hydrogen ion concentration. A buffer is a substance that can accept or donate hydrogen ions to resist a change in pH.

At a pH away from the optimum, H⁺ ions disrupt the hydrogen and ionic bonds that maintain the tertiary structure of the enzyme. This causes the enzyme to denature and the active site to change shape so it is no longer complementary to the substrate. The H⁺ ions may also associate with certain amino acid R groups at the active site altering the charge distribution and prevent substrate binding. The means fewer ESC form and the rate of reaction is slow.
Not all enzymes have the same optimum pH. In general, most enzymes have a pH of around 7, however enzymes that act in the stomach in stomach acid, such as pepsin, have a lower optimum pH because their tertiary structure is maintained by fewer hydrogen and ionic bonds. The pH of blood must be maintained by homeostasis as changes in pH can denature proteins and cause vasodilation/vasoconstriction.
Enzyme concentration
As enzyme concentration increases, more active sites are available to bind to the substrate resulting in more frequent collisions between the active site and substrate, therefore more ESC form increasing the initial rate of reaction. At this point all the active sites are occupied as there is excess substrate. However, eventually the rate of reaction reaches a Vmax as, substrate concentration becomes the limiting factor and some active sites are left unoccupied.
Substrate concentration
As substrate concentration increases, there will be more frequent collisions between the active sites and substrate, therefore more ESC form increasing the initial rate of reaction. At this point not all active sites are occupied as enzymes are in excess. However eventually the rate of reaction reaches a Vmax as enzymes concentration becomes the limiting factor and all active sites are occupied.