Sturcture & Function of the Nervous System
The Neuron
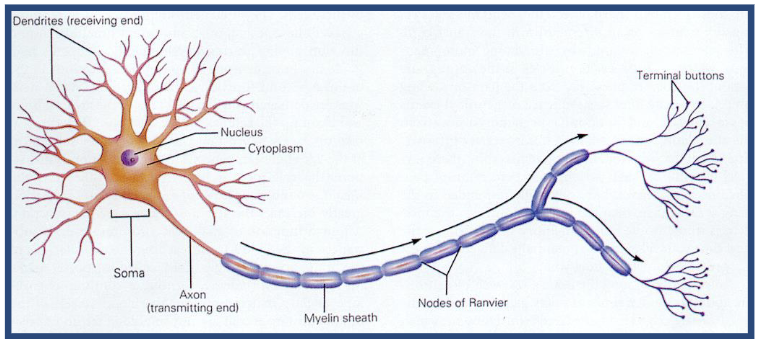
Dendrites
Some/Cell body
Nucleaus
Axon Terminal button
Myelin
The Need for Ion Channels
Ion channels are membrane proteins
Membrane impermeable to ions
ion channels or ionophores allow some ions through
Leaves a resting potential of -70 mV
Permeability determines resting potential
Ion Channels:
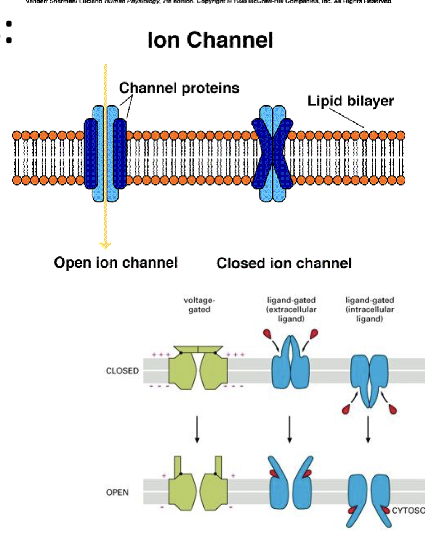
Two Types:
1) Gated
Ligand-gated
Voltage-gated
2) Non-Gated
Ions flow along concentration gradients
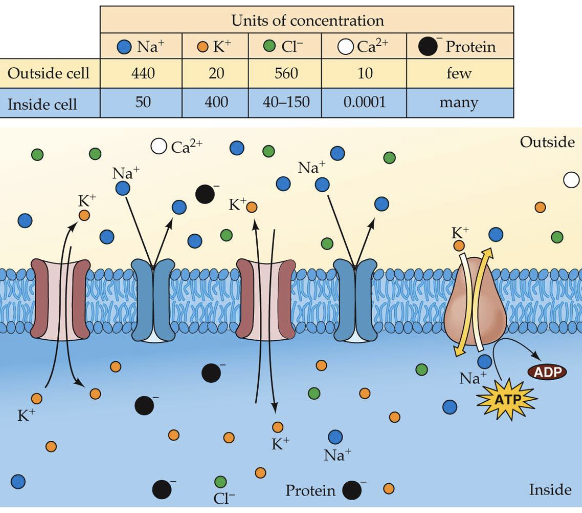
Resting Potential
-70mV
Where does the resting potential come from?
Membrane has a negatively charged inner lining of relatively large proteins with a negative charge called A-ions (anions).
Elemental Ions:
K+ (Potassium) - does most of the movement
Na+ (sodium)
Cl- (chloride)
CA++(calcium)
Why the uneven distribution of ions?
Na+ /K+ ion pump
– 2 K+ pumped in for every 3 Na+ pumped out
– more + ions outside than insideOther forces:
– Concentration gradient (diffusion)
– Electrostatic gradientDistribution of ions inside and outside a neuron at resting potential
Stimulation of the Action Potential
Local Potential
Depolarization
Hyperpolarization
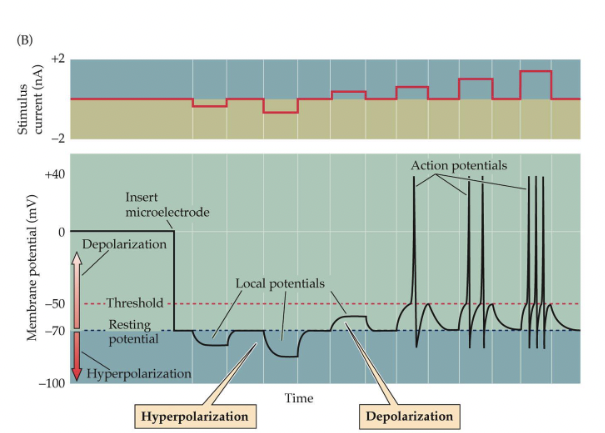
Action Potential:
Occurs when a neuron is depolarized past threshold for firing (-55 mV)
Voltage-gated Na+ ion channels open, and ions flow in along diffusion and electrostatic gradients.
K+ channels then open, allowing K+ ions to flow out
Gated close and ion pumps restore normal resting potential
All-or-None Law
Post-synaptic potentials are generated in dendrites and cell bodies.
Action potentials move along the membranes of a neuron. They are initiated at the axon hillock and travel along axons away from the cell body.
How does activity in one neuron influence activity in another?
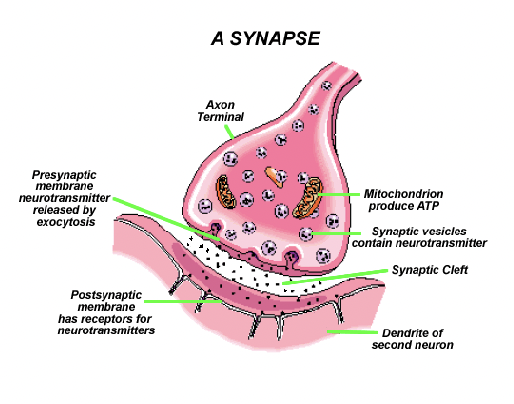
Syanpsses
Presynaptic Neuron:
terminal bouton at the end of axons
Synaptic Cleft
Post-synaptic Neuron:
receptor sites
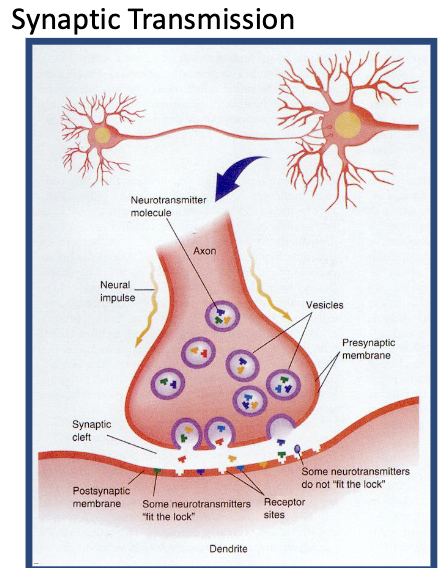
Activity at Synapses
Arrival of an action potential causes Ca++ ion channels to open, facilitating the release of a neurotransmitter.
A neurotransmitter binds to receptor sites, causing a change in the resting potential of the membrane.
Transmitter action is terminated by reuptake or enzymes.
Post-Synaptic Action
May directly (gated ion channel) or indirectly (second messenger) open ion channels.
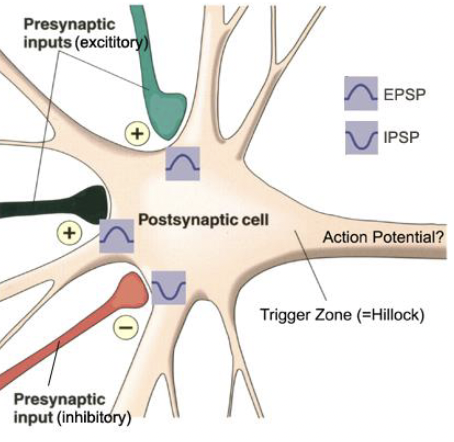
Depolarization causes excitation (opens Na+ channels) and increases firing rate. (EPSP)
Hyperpolarization causes inhibition (opens Cl - channels) and decreases firing rate. (IPSP)
Summation of Excitation & Inhibition
Spatial
Temporal
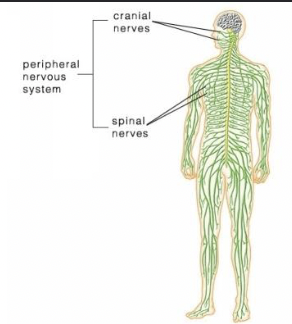
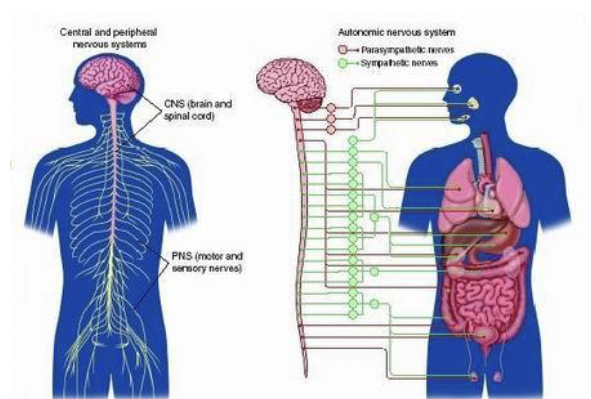
The Nervous System
CNS - brain and spinal cord
PNS - everything else
Bundles of axons
white matter - nerves (PNS) or tracts (CNS)
Clusters of cell bodies (somata)
ganglia (PNS) or centres (nuclei) (CNS)
PNS - Peripheral Nervous System
Somatic
Conscious senses (sensory afferents)
Voluntary motor (motor efferents)
Autonomic
Sympathetic NS - fight or flight; epinephrine (E)
parasympathetic NS - vegetative functions;
Acetycholine (ACh)
Drugs that alter E or ACh could affect ANS functioning
CNS and PNS
CNS - Central Nervous System
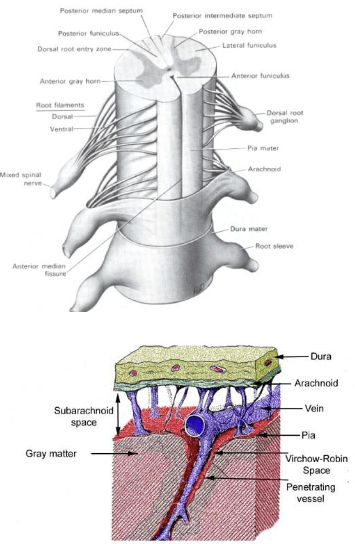
Spinal cord
relay centre
reflexes
forsal - sensory input (afferents)
ventral - motor output (efferents)
Brain
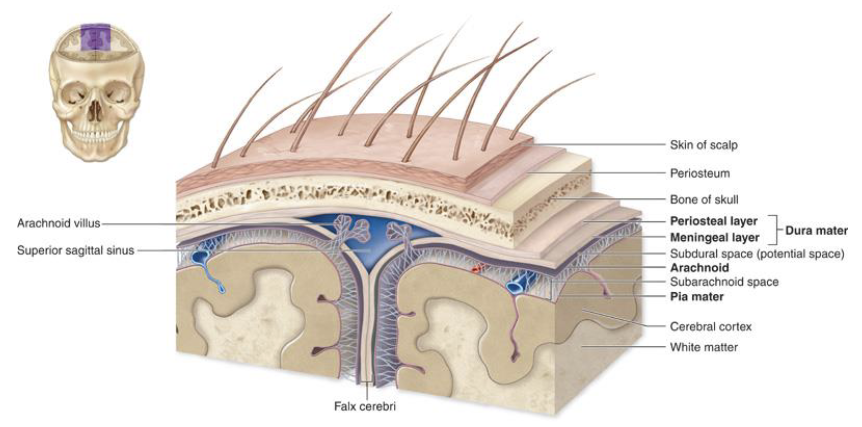
3 layers of tissue protection (meninges)
Dura mater
Arachnoid
Pia mater
The Meninges
The sequence in describing neuronal transmission?
Dendrites - soma - axon hillock - axon - terminals.
Dendrites: These branching, tree-like structures receive incoming signals from other neurons.
Soma: Also known as the cell body, it integrates the incoming signals from the dendrites.
Axon hillock: This is the junction between the soma and the axon. If the integrated signals exceed a certain threshold at the axon hillock, an action potential is generated.
Axon: The long, slender projection that transmits the electrical signal (action potential) away from the cell body toward the terminals.
Terminals: Located at the end of the axon, these structures form synapses with the dendrites or cell bodies of other neurons, allowing the transmission of signals between neurons.
Glial Cells
Gilial Cells Provide Vital Support for Neurons
Schwann cells: form myelin sheaths in the PNS, wrap one axon, and promote regeneration of damaged axons.
Oligodendroglia: form myelin sheaths in the CNS; wrap around many axons (see above in B); do not have nerve growth factors for regeneration.
Astrocytes: structural support for neurons; modulate the extracellular environment; take up excess neurotransmitters to reduce cell damage.
Microglia: remove dying cells by phagocytosis at sites of nerve damage; source of immune response in the CNS.
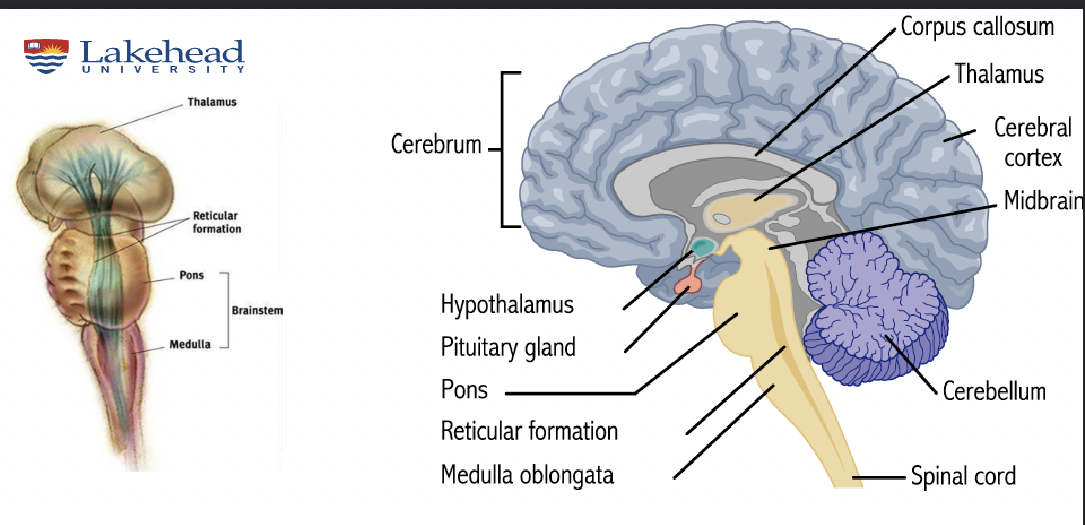
Medulla
breathing
vomiting (area postrema)
Reticular Activating System (RAS)
arousal
contains locus coeruleus (norepinephrine)
contains Raphe nuclei - sleep & mood (serotonin)
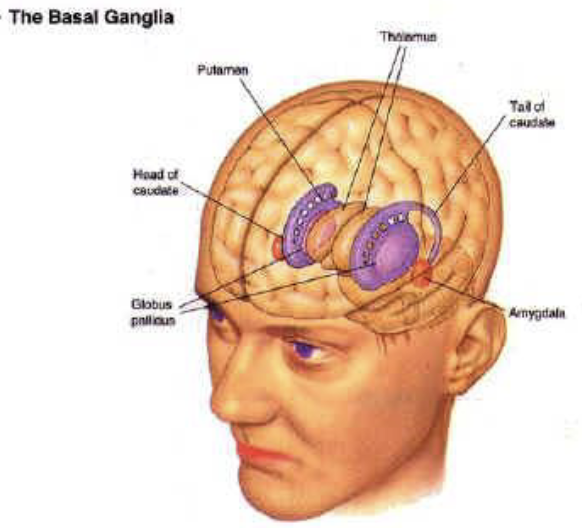
Basal Ganglia
Striatum (caudate nucleus & putamen)
input from the thalamus and cortex
Globus Pallidus
output side with feedback to the thalamus
Coordination of Motor Control
Extrapyramidal motor system
Dopamine (DA) receptors
DA Deficiency - Parkinson’s Disease
Periaqueductal Grey (PAG)
Pain control: mu receptors and morphinelike transmitters
also involved in defensive behaviour and predation (species’ typical behaviours)
Limbic System
Hypothalamus
easting & drinking control; emotions; motivation
Medial Forebrain Bundle
reinforcement centres
mesolimbic system (DA) -ventral tegmental area (VTA)
Nucleus accumbens
Hippocampus
learning & memory (HM)
Personal memory & spatial memory
Cingulate Cortex
Social behaviour (e.g., sexual), decision-making, and executive functions
Amygdala
Serotonergic input from the Raphe system
Aggression & emotion
Cortex
Sensory input areas
Motor control output areas
Language
Memory & thinking
Glutamate - excitatory transmitter
GABA - inhibitory transmitter
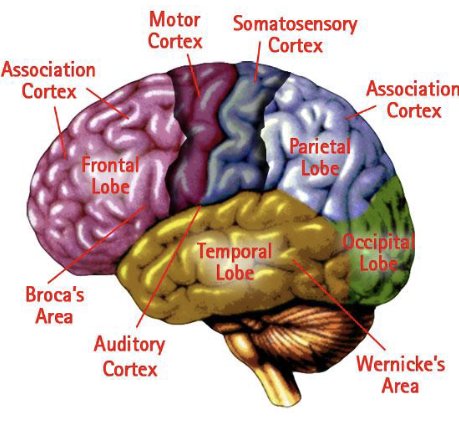
Cerebrum / 4 lobes of the brain
Frontal lobe
Behind the forehead
Control of voluntary muscles
Cognition/Reasoning/EFs
Parietal lobe
Top of the head, toward the rear
Processing bodily sensations
Phineus Gage
A large iron rod was driven completely through his head, destroying much of his brain’s left frontal lobe.
Friends described him as “no longer Gage.” (personality and behaviour changes)
Cerebrum / Neocortex
Development of the Nervous System
Cells formed during the first 12 weeks
Migrate to the appropriate location along radial glial cells (“roads”) due to chemical signals.
Send out axons to correct the target
Form synapses
Some cortical neurons also migrate by following only chemical signals
Psychoactive drugs can disrupt the process because:
Get into the brain
Confuse or block chemical signals
Drugs are likely to disrupt the chemical signals in the fetus because:
Drugs pass through the placenta fairly easily (diffusion)
More blood circulates to the fetus's brain than to the adult brain (thus more drug exposure) (also due to weaker BBB)
Fetus has fewer plasma proteins for binding, thus more drug binds to the brain
Fetus’ ability to metabolize drugs is usually lower.
Teratology:
Studies of abnormal physiological development (including brain malformation)
Examples: Fetal Alcohol Syndrome, Thalidomide
Functional or Behavioural Teratology
there may be no anatomical malformations, but the functioning of the brain may be disturbed
there may be behavioural changes
Stages of Protein Synthesis
Transcription: Production of complementary mRNA from coding regions of DNA; takes place in the nucleus.
Translation: Building of protein molecules by linking amino acids specified by the mRNA blueprint; takes place on ribosomes in the cytoplasm.
Epigenetic Effects
Epigenetics: control of gene expression by chromosome modifications (e.g., methylation, acetylation) that do not affect the DNA code.
Environmental events (e.g., foods, drugs, stress) can increase of decrease transcriptiom factors that increase or decrease the likelihood of proteins being created.
Genes can be turned off long-term, and the activation or inactivation of certain genes can be passed on to offspring if this occurs in the cells of eggs or sperm.
Thus, behaviour affects your genes now and later…
DNA methylation: attachment of methyl groups to a gene reduces its expression (blocks translation).
Modification of histone tails makes chromatin more tightly packed, which represses gene expression by physically limiting the access of transcription factors.
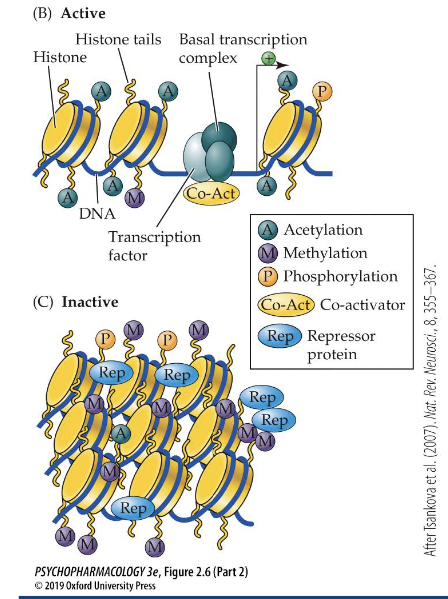
Epigenetic regulation of gene transcription
Acetylation - opens up and activates the gene for transcription ( See B)
Methylation - tightens up the gene, stopping transcription (See C)
Epigenetic modification of gene expression is central to cell differentiation in the developing fetus.
Epigenetic changes can be passed on to offspring if they occur in germ-line cells.
Various studies suggest that parents’ lifelong environmental exposure can influence their offspring’s behaviour, metabolism, or disease status.
Many epigenetic differences are related to environmental factors (diet, stress, drug-taking)
Epigenetics may explain some phenomena, such as;
Why do monozygotic twins with identical genes not always develop the same disorders?
Persistence of the drug-taking behaviour characteristics of addiction.
The link between early abuse or neglect and clinical depression.
Neurotrophic Factors
Proteins that influence neuron growth, cell differentiation and sociaval, and synaptic connections.
NGF (nerve growth factor): secreted by peripheral organs; guides the growth of axons to establish synapses at target organs.
BDF (brain-derived neurotrophic factor): in the CNS; important in development and in the adult brain, a key factor in learning and memory; helps neurons.
Gliial Schwann cells (form muelin) release a growth factor to regenerate damaged axons.
Neurotrophic factors are potential therapeutic agents for neurodegenerative diseases and psychiatric disorders.