Unit 4.1 Ionic & Covalent Bonding
4.1.1 Forming Ions
Metals are on the left of the Periodic Table
Lose electrons from their valence shell
Form positively charged cations
Non-metals are on the right of the Periodic Table
Gain electrons
Form negatively charged anions
Ionic Bonds = bonds formed from the transfer of electrons from a metallic element to a non-metallic element
Usually results in both the metal and non-metal having a full outer shell
Thus, these atoms have electronic configurations that are the same as a noble gas (elements with full outer shells)
Example:
Na (Sodium)
It is a metal in group 1, so it has 1 valence electron
After it loses this electron, it becomes Na+ (Sodium Cation)
Cation = A positively charged ion
A sodium ion has the same electronic configuration as neon: [2, 8]
CL (Chlorine)
It is a non-metal in group 17, so it has 7 valence electrons
After it gains an electron from a different atom, it becomes Cl- (Chloride Anion)
Anion = A negatively charged ion
A chloride ion has the same electronic configuration as argon: [2, 8, 8]
Electrostatic Attractions = Attractions formed between the oppositely charged ions (cations and anions) to form ionic compounds
Very strong, so it takes a lot of energy to break
This is why ionic compounds have high melting points
4.1.2 Ionic Compounds
Ionic Lattices
Ionic Lattice = an evenly distributed crystalline structure formed by ions
Ions arranged in a regular repeating pattern due to electrostatic forces of attraction between cations and anions
This causes positive charges to cancel out negative charges, causing the final, overall lattice to be electrically neutral
Properties of Ionic Compounds
Different types of structure/bonding → different physical properties (ie. melting/boiling points, electrical conductivity, and solubility)
Ionic Bonding & Giant Ionic Lattice Structures
Ionic compounds are strong
Their strong electrostatic forces keep ions held together strongly
Ionic compounds are brittle, so ionic crystals can split apart
Ionic compounds have high melting and boiling points
This is because of their strong electrostatic forces between the ions that keep them held strongly together
As charge density of the ions increase, the melting and boiling points increase
This is due to the greater electrostatic attraction of charges
ex. Mg2+O2- has a higher melting point than Na+Cl-
Ionic compounds are soluble in water because they can form ion-dipole bonds
Ionic compounds only conduct electricity when molten or in solution
In those two cases, the ions can freely move around and conduct electricity
However, as a solid, the ions are fixed, so they are unable to move around
4.1.3 Formulae & Names of Ionic Compounds
Review:
Ionic compounds are formed from a metal and a nonmetal bonded together
Ionic compounds are electrically neutral (positive charges = negative charges)
Charges on Positive Ions
Positive Ions:
All metals
Some non-metals
ex. NH4+ (Ammonia) and H+ (Hydrogen)
Charges of ions depend on their position in the Periodic Table:
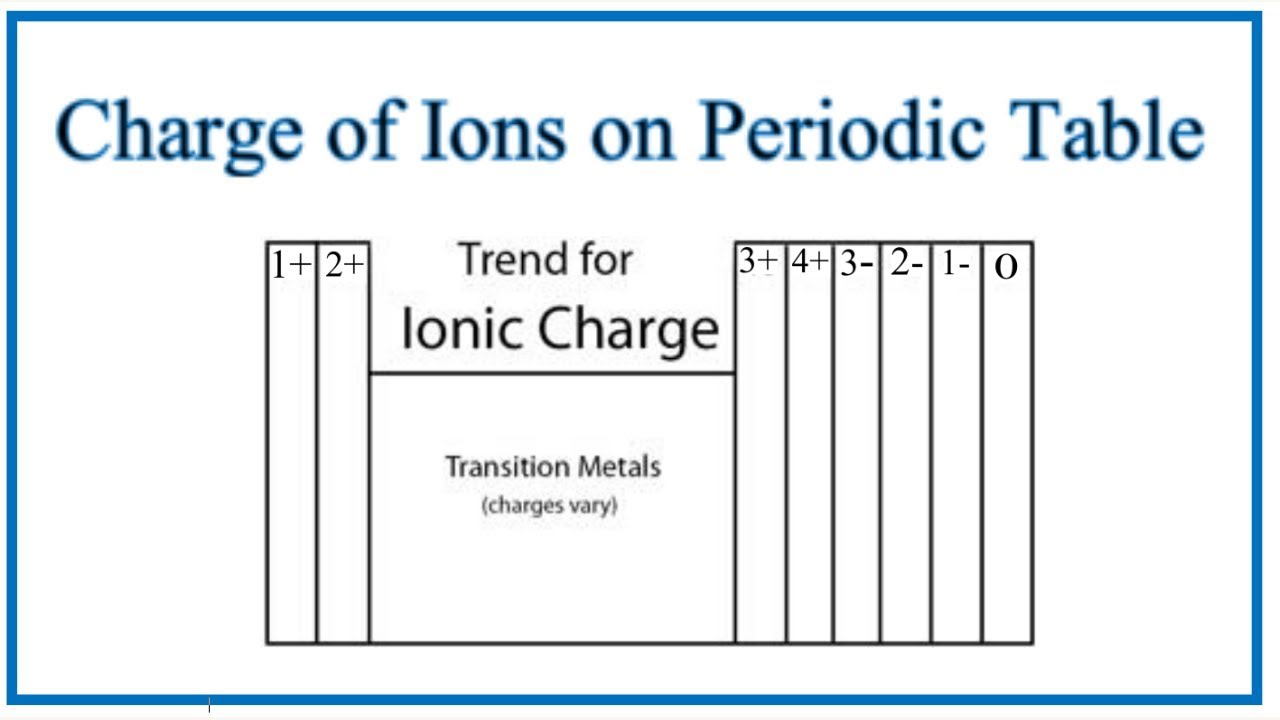

Metals in Groups 1, 2, and 13 = charges of 1+, 2+, and 3+
Charge on ions of the transition metals can vary which is why Roman numerals are often used to indicate their charge
ex. Copper (II) Oxide = copper ion has a charge of 2+
ex. Copper (I) Nitrate = copper ion has a charge of 1+
Non-metal Ions
Non-metals in Groups 15-17 have a negative charge and have the suffix “-ide”
ex. Nitride, Chloride, Bromide, Iodide
Elements in Group 17 = gain 1 electron → have a 1- charge
ex. Br-
Elements in Group 16 = gain 2 electrons → have a 2- charge
ex. O2-
Elements in Group 15 = gain 3 electrons → have a 3- charge
ex. N3-
Additionally, there are more polyatomic or compound negative ions (negative ions made up of more than one type of atom)
7 Common Polyatomic Ions
Ion | Formula and Charge |
|---|---|
Ammonium | NH4+ |
Hydroxide | OH- |
Nitrate | NO3- |
Sulfate | SO42- |
Carbonate | CO32- |
Hydrogen Carbonate | HCO3- |
Phosphate | PO43- |
4.1.4 Covalent Bonds
Covalent Bonds
Covalent Bonding occurs between 2 non-metals
Involves the electrostatic attraction between the nuclei of 2 atoms and the electrons of their outer shells
No electrons transferred, but only shared
2 atomic orbitals overlap and a molecular orbital is formed (see left side of image, “bonding”)
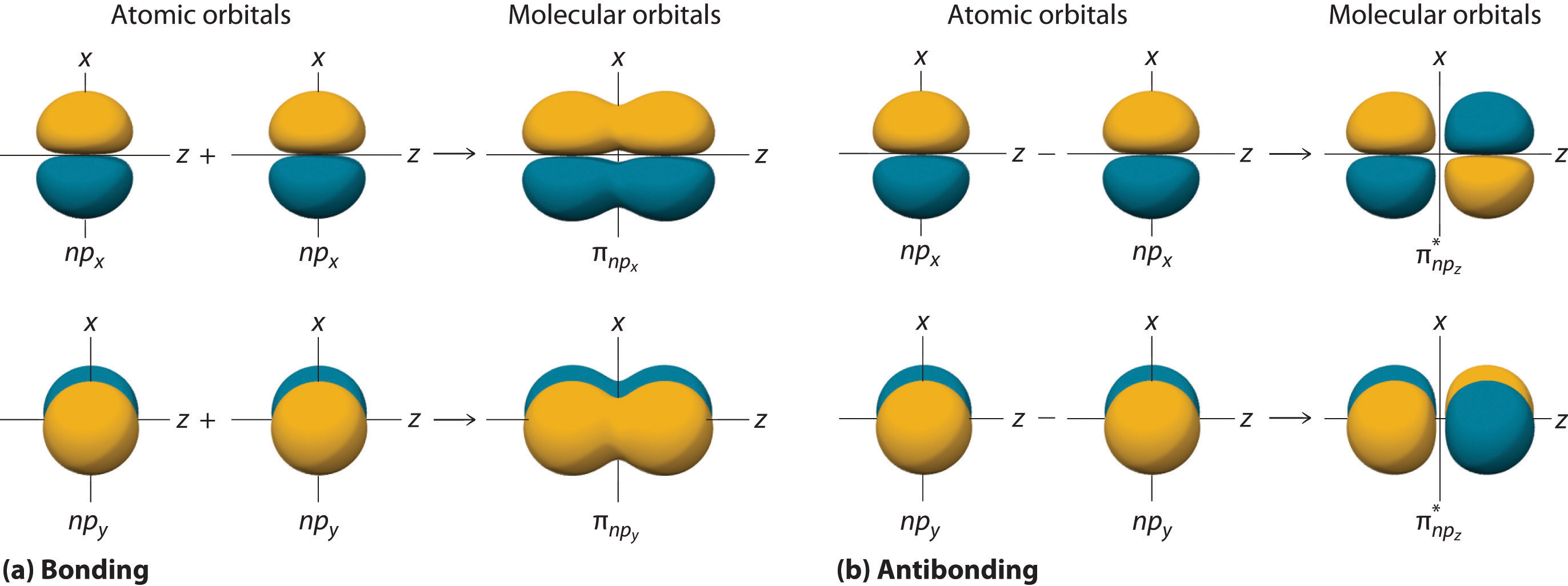
Covalent bonding occurs due to the fact that electrons are more stable when attracted to 2 nuclei rather than only 1
Why are they more stable?
Because sharing electrons allows each of the atoms to achieve an electron configuration similar to a noble gas (octet rule)
Usually, each atom provides one of the electrons in the bond
A covalent bond is represented by a short straight line between 2 atoms
ex. H-H
Note: Think about covalent bonds as electrons being in a constant state of motion, or “charge clouds” rather than an electron pair in a fixed position
Predicting Covalent Bonding
Use differences in electronegativity to predict whether a bond is either covalent or ionic
Electronegativity & Covalent Bonds
Electron density in diatomic molecules are shared equally
ex. H2, O2, Cl2
Difference in Electronegativity | Bond Type |
|---|---|
< 1.0 | Covalent |
1.0 - 2.0 | Polar Covalent |
> 2.0 | Ionic |
Coordinate Bonds
In simple covalent bonds, the 2 atoms involved share electrons
However, some molecules have a lone pair of electrons that can be donated to form a bond with an electron-deficient atom (an atom that has an unfilled outer orbital)
So both electrons are from the same atom
This type of bonding is called dative covalent bonding or coordinate bond
An example of a dative bond is in an ammonium ion
The hydrogen ion, H+ is electron-deficient and has space for two electrons in its shell
The nitrogen atom in ammonia has a lone pair of electrons which it can donate to the hydrogen ion to form a dative covalent bond
Multiple Bonds
Non-metals are able to share more than one pair of electrons to form different types of covalent bonds
Sharing electrons in the covalent bond allows each of the 2 atoms to achieve an electron configuration similar to a noble gas
This makes each atom more stable
It is not possible to form a quadruple bond as the repulsion from having 8 electrons in the same region between the two nuclei is too great
Type of Covalent Bond | # of electrons shared |
|---|---|
Single (C - C) | 2 |
Double (C = C) | 4 |
Triple (C ≡ C) | 6 |
Bond Length & Strength
Bond Energy
Bond Energy = The energy required to break one mole of particular covalent bond in the gaseous states (in units of kJ mol-1)
The larger the bond energy, the stronger the covalent bond is
Bond Length
Bond length = internuclear distance of two covalently bonded atoms
It is the distance from the nucleus of one atom to another atom which forms the covalent bond
The greater the forces of attraction between electrons and nuclei, the more the atoms are pulled closer to each other
This decreases the bondlength of a molecule and increases the strength of the covalent bond
Triple bonds are the shortest and strongest covalent bonds due to the large electron density between the nuclei of the two atoms
This increase the forces of attraction between the electrons and nuclei of the atoms
As a result of this, the atoms are pulled closer together causing a shorter bond length
The increased forces of attraction also means that the covalent bond is stronger

Triple bonds are the shortest covalent bonds so they are the strongest
4.1.5 Bond Polarity
When two atoms in a covalent bond have the same electronegativity the covalent bond is nonpolar (In other words, the difference in electronegativity = 0)
When two atoms in a covalent bond have different electronegativitiesthe covalent bond is polar and the electrons will be drawn towards the more electronegativeatom
As a result of this:
The negative charge center and positive charge center do not coincidewith each other
This means that the electron distributionis asymmetric
The lesselectronegative atom gets a partial charge of δ+ (deltapositive)
The moreelectronegative atom gets a partial charge of δ- (deltanegative)

The greater the difference in electronegativity the more polar the bond becomes
Dipole moment
The dipole moment is a measure of how polar a bond is
The direction of the dipole moment is shown by the following sign in which the arrow points to the partially negatively charged end of the dipole:

4.1.6 Lewis Structures
Lewis structures are simplified electron shell diagrams and show pairs of electrons around atoms.
A pair of electrons can be represented by dots
 The Octet Rule = The tendency of atoms to gain a valence shell with a total of 8 electrons
The Octet Rule = The tendency of atoms to gain a valence shell with a total of 8 electrons
Steps for drawing Lewis Structures
Count the total number of valence
Draw the skeletal structure to show how many atoms are linked to each other.
Use a pair of crosses or dot/cross to put an electron pair in each bond between the atoms.
Add more electron pairs to complete the octets around the atoms ( except H which has 2 electrons)
If there are not enough electrons to complete the octets, form double/triple bonds.
Check the total number of electrons in the finished structure is equal to the total number of valenceelectrons
Incomplete Octets
For elements below atomic number 20 theoctet rule states that the atoms try to achieve 8 electrons in their valence shells, so they have the same electron configuration as a noble gas
However, there are some elements that are exceptions to the octet rule, such a H, Li, Be, B and Al
H can achieve a stable arrangement by gaining an electron to become 1s2, the same structure as the noble gas helium
Li does the same, but losing an electron and going from 1s22s1 to 1s2 to become a Li+ ion
Be from group 2, has two valence electrons and forms stable compounds with just four electrons in the valence shell
B and Al in group 13 have 3 valence electrons and can form stable compounds with only 6 valence electrons
