Chemistry Honors Finals
Unit 6 Ionic Bonding Highlights
- Symbol- a one, two, of three letter designation for an element (Ex. Sodium is Na)
- Subscript- a number written to the right and below a symbol that tells the number of atoms present in a molecule (Ex. An oxygen molecule has two oxygen atoms, O2)
- Formula- symbols and subscripts used to represent the composition of a substance (can be ionic or covalent (Ex. H2O, KCl)
- Chemical Bonds- forces that hold atoms together
- protons of one atom and electrons of another are attracted
- Ionic bonds- between metals and nonmetals, they transfer electrons, metals are losers (lose electrons) and nonmetals gain electrons
- Form an ionic compound (a salt), ions stick together like magnets
- Mostly crystalline solids due to high boiling and melting points
- Conduct electricity when in the liquid or dissolved state (needs mobile ions)
- Compound- a substance composed of 2 or more elements chemically combined in definite proportions
- Ex. MgCl2 is a compound but Br2 is NOT
- Compounds can be broken down by chemical means (unlike pure elements that cannot)
- Polyatomic Ion- a group of covalently bonded atoms possessing a charge, they take part in ionic bonding (Ex. NH4+, CO3-2)
- A compound can have both covalent and ionic bonding if it has a polyatomic ion
Ex. BaCO3, Ca(OH)2, NH4F
- Writing Ionic Formulas: Criss-Cross Method
- Ex. Mg+2Cl-1 🡪 MgCl2
- Ex. Ca+2NO3-1 🡪 Ca(NO3)2
- (Polyatomic ions need parentheses if there is more than one)
- Ex. Be+2O-2 🡪 Be2O2 🡪 BeO
- (Always reduce to most reduced form)
- Naming Ionic Compounds:
- Metal named first, change ending of nonmetal to “-ide”
- Ex. NaF 🡪 Sodium Fluoride
- Transition Metals with more than one possible charge need a Roman Numeral to tell the charge of the metal atom
- Ex. MnI2 🡪 Manganese (II) iodide
- You can find the charge of the metal by using Reverse Criss-Cross if subscripts are present OR by checking the charge of the anion
- Ex. Fe2O3 🡪 Fe+3O-2 🡪 Iron (III) oxide
- Ex. CuSO4 🡪 Cu+2(SO4)-2 🡪 Copper (II) sulfate
- Ex. AuF 🡪 Au+1F-1 🡪 Gold (I) fluoride
- Metal named first, change ending of nonmetal to “-ide”
- Lewis Dot Diagram- place number of valence electrons around the element’s symbol, always placing two on top first, then one on each side as you draw them
- Ex.

- Lewis Diagrams- every atom needs its octet
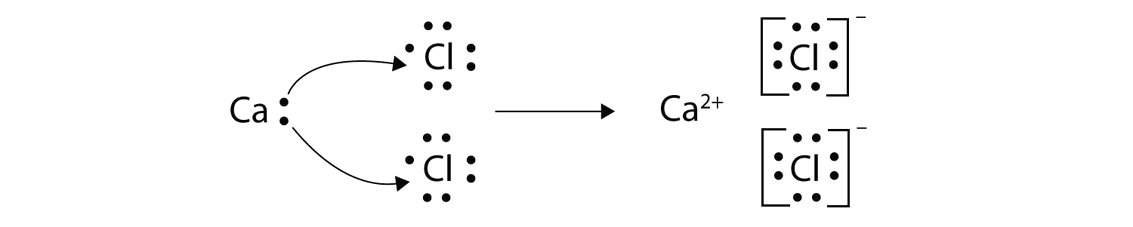
- Ionic- metals give all electrons to nonmetal, forms ions, write brackets and charges, nonmetals should have 8 electrons, metals should have none
- Ex.:
- Ionic- metals give all electrons to nonmetal, forms ions, write brackets and charges, nonmetals should have 8 electrons, metals should have none
Unit 7 Highlights: Covalent Bonding
- Covalent bonds- between nonmetals, share valence electrons to give all atoms octets
- Form a molecule
- Poor conductors of heat and electricity
- Mostly gases, liquids and softer solids because of lower boiling and melting points
- Single Bond 🡪 2 e-, Double Bond 🡪 4 e-, Triple Bond 🡪 6 e-
- Lewis Dot Diagram- place number of valence electrons around the element’s symbol, always placing two on top first, then one on each side as you draw them
*Except Carbon, one dot each side
- Lewis Diagrams- every atom needs its octet
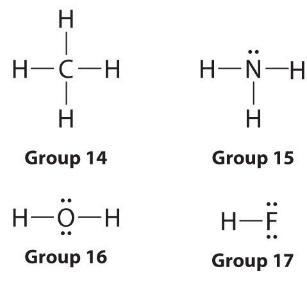
- Covalent- connect lone dots so as to complete octets, redraw shared electrons as lines to represent bonds
- Ex.:
- Covalent- connect lone dots so as to complete octets, redraw shared electrons as lines to represent bonds
- Polarity- a measure of electron distribution within a molecule (covalent only because they share e-), is determined by the difference in electronegativity between atoms
- Polar- uneven distribution of electrons, different electronegativities (different elements), do not share electrons equally
- One atom is more negative than the other because it pulls the electrons towards it (Ex. HCl, H2O, PCl3, NH3)
- WATER IS POLAR!!!!!!
- Nonpolar- even distribution of electrons, same electronegativity (same element), share electrons equally (Ex. H2, O2)
- Polar- uneven distribution of electrons, different electronegativities (different elements), do not share electrons equally
- VSEPR (Valence Shell Electron Pair Repulsion)
- Molecular shape depends on the pairs of valence electrons around the central atom which repel each other
- Electron pairs will arrange themselves to be as far apart from each other as possible
- Repulsive unit: a single bond, double bond, triple bond or lone pair - will try to get as far away from other repulsive units as possible
- Molecular shape depends on the pairs of valence electrons around the central atom which repel each other
Name of shape | # of atoms bonded to central atom | # of lone pairs on central atom | # of repulsive units | Bond angle | 3D shape |
|---|---|---|---|---|---|
Linear | 2 | 0 | 2 | 180o | 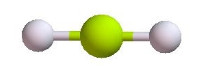 |
Trigonal Planar | 3 | 0 | 3 | 120o | 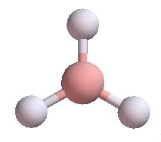 |
Bent or V-shaped | 2 | 2 | 4 | 105o | 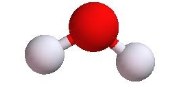 |
Trigonal Pyramid | 3 | 1 | 4 | 107o | 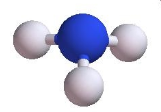 |
Tetrahedral | 4 | 0 | 4 | 109.5o | 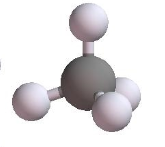 |
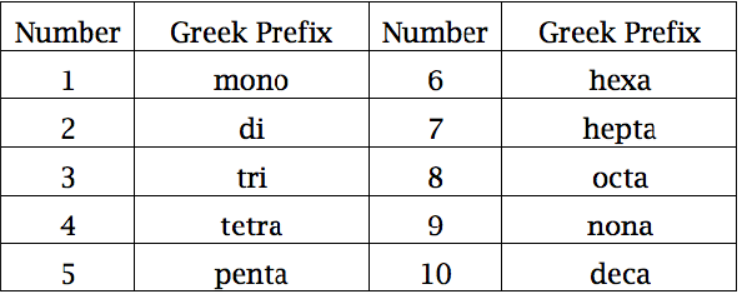
- Naming Covalent Compounds
- First element stays the same, second ends in “-ide”
- Use prefixes for both to tell the number present (never use “mono-” for the first)
- Ex. CBr4 🡪 Carbon tetrabromide
- Ex. N2O3 🡪 Dinitrogen trioxide
- Ex. CO 🡪 Carbon monoxide
Unit 8 Highlights
- Physical Change- a change in state (Gas, liquid, solid) or physical appearance (the substance stays the same from beginning to end)
- Ex. CH4(g) 🡪 CH4(l)
- Chemical Change- then the identity of the products is different from the identity of the reactants (a chemical reaction, the substance changes)
- Ex. 2H2(g) + C(s) 🡪 CH4(g)
- Signs a Reaction has occurred
- Color change
- Bubbles form (gas released)
- Temperature change
- Odor (smell)
- Formation of a precipitate (insoluble solid)
- Reactant- a substance that enters a reaction, on the left side of the equation
- Product- a substance formed in a reaction, on the right side of the equation
- Reactants 🡪 Products
- Coefficient- a number written in front of a formula to tell how many substances or molecules there are (Ex. 2NH3 means two NH3 molecules)
- Five main types of chemical Reactions:
- Synthesis (Combination) Reaction- A + B → AB
- (Ex. 2H2 + O2 → 2H2O)
- Decomposition Reaction- AB → A + B
- (Ex. CaCO3 → CaO + CO2)
- Single Replacement Reaction- A + BC → B + AC
- (Ex. Zn + CuI2 → Cu + ZnI2)
- Double Replacement Reaction- AB + CD → AD + CB
- (Ex. KBr + MgO → K2O + MgBr2)
- Combustion Reaction - CxHy + O2 → H2O + CO2
- (Ex. CH4 + 2O2 → 2H2O + CO2)
- Synthesis (Combination) Reaction- A + B → AB
- Law of Conservation of Mass: states that matter AND energy are both conserved in a chemical reaction (charge is also conserved).
- This implies that reactions must be balanced
- To balance a reaction one must modify coefficients to make sure that the number of atoms of each element on both sides of the reaction are equal
- Ex. ___Na + ___Cl2 🡪 ___NaCl
2Na + Cl2 🡪 2NaCl
(2 Na atoms and 2 Cl atoms on both sides)
- Ex. ___AlBr3 + ___K2SO4 → ___KBr + ___Al2(SO4)3
2AlBr3 + 3K2SO4 → 6KBr + Al2(SO4)3
(2 Al atoms, 6 Br atoms, 6 K atoms, 3 SO4 ions)
Unit 9 Highlights
- Gram Formula Mass (GFM)- the sum of the atomic masses of all atoms present in a compound, this is also the mass of one mole of that compound
- Ex. H2O = 2(H) + O = 2(1) + 16 = 18g/mol
- H2O, the GFM is 18g, meaning 1 mole of water weighs 18g, 2 moles would weigh 36g, etc.
- Percent Composition by Mass = (mass of part) / (mass of whole) × 100
- Remember: if there is more than one atom of the element being calculated as the part, all atoms must be accounted for in the numerator as in the example below
- Ex. For H2O: 2(H) + O = 2(1) + 16 = 18g/mol
- %Mass of H = 2(1)/18 × 100 = 11.1% H
- %Mass of O = 16/18 × 100 = 88.9% O
- Empirical Formula: simplest whole number ratio of elements in a compound (always used for ionic compounds)
- To find empirical formula from percent composition
- Imagine a 100g sample, percentages represent grams in this sample
- Convert grams to moles by dividing by the GFM of that element
- Compare ratios of moles to find empirical formula
- Ex. 36% Ca, 64% Cl
- 36/40 = 0.9, 64/35.5 = 1.8
- 0.9:1.8 = 1:2, CaCl2
- To find empirical formula from percent composition
- Molecular formula: actual ratio of elements in a compound
- To find the molecular formula given the GFM and the empirical formula, divide the GFM of the molecular formula by the GFM of the empirical formula and multiply each subscript in the empirical formula by this value
- Ex. What is the molecular formula of a compound with an empirical formula of C2H4O and a GFM of 88 g/mol.
- GFM of empirical formula = 12(2) + 1(4) + 16 = 44
- 88/44 = 2
- C2x2H4x2O2x1 = C4H8O2
- To find the molecular formula given the GFM and the empirical formula, divide the GFM of the molecular formula by the GFM of the empirical formula and multiply each subscript in the empirical formula by this value
- Mole- a unit used to tell how much of a substance there is, 1 mole = 6.02 × 1023 atoms or molecules (Avogadro’s Number) and 1 mole of gas occupies 22.4 L at STP (standard temperature and pressure)
- The GFM of an element or formula tells how much 1 mole of that substance weighs in grams… these are the numbers on the periodic table for Atomic Mass!
- Ex. 1 mole of He atoms weighs 4g
- Ex. 1 mole of H2O2 molecules weighs 2(1) + 2(16) = 34g
- Converting Moles and Grams: Moles = Grams / (GFM)
- Step 1: Find the GFM of the element or compound
- Step 2: Plug in to the equation and solve
- Ex: Calculate how many moles of molecules are present in 4.75g of F2. (GFM = 38 g/mol)
- Moles = 4.75g/38 = 0.125 mol
- Ex: Calculate how many grams 4.3 mol of Mn will weigh.
- 4.3mol = x/55, x = 236.5g
Unit 10 Gas Laws Highlights
Kinetic Molecular Theory (KMT)
- Gas particles are in random, straight line motion
- Collisions between particles are elastic, energy is transferred from one particle to another but overall momentum is conserved
- Gas particles are separated by large distances relative to the size of the actual gas particles, therefore the size of the gas particles is negligible
- There is no attractive force between particles
- Temperature is a measure of the average kinetic energy of particles, higher temperature means faster moving particles
- Collisions with walls of container and other particles creates pressure
Gas Variables
- Pressure (P): how hard and how often the particles collide with each other and the walls, (Force/area) [atm, kPa, torr, mmHg, bar, psi]
- Volume (V): space occupied by the gas, [L, mL, cc, m3]
- Temperature (T): measure of the average kinetic energy of the particles, [K, oC] (must convert to K,
K = oC + 273)
- Amount of gas (n): number of moles of gas, [mol]
Gas Constant
- R: value depends on the units for pressure
- P in atm, R = 0.082 Latm/molK P in kPa, R = 8.314 J/molK
Gas Laws
- Boyle’s Law: Pressure and volume are inversely related, P1V1 = P2V2
- Charles’ Law: Temperature and volume are directly related, V1/T1 = V2/T2
- Gay-Lussac’s Law: Pressure and temperature are directly related, P1/T1 = P2/T2
- Avogadro’s Law: Number of moles and volume are directly related, n1/V1 = n2/V2
- Combined Gas Law:
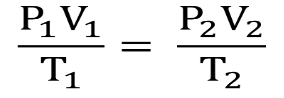
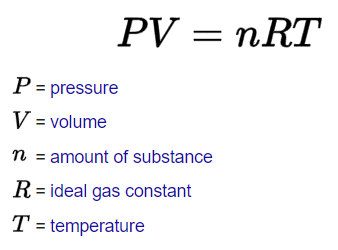
- Ideal Gas Law:
Unit 11 Highlights: Solutions
- Solution- a homogeneous mixture (uniform, it looks like one thing even though it isn’t)
- clear and do not disperse light
- can have color
- solute will not settle upon standing (once solute is dissolved it stays dissolved)
- will pass through a filter (the filter will not separate solute and solvent)
- Solute- the substance being dissolved (ex. Salt, sugar)
- Solvent- the substance doing the dissolving (usually water)
- (aq)- means aqueous, dissolved in water…is a mixture and a solution
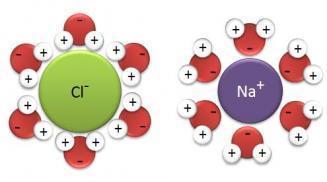
- “Like dissolves like”- polar solvents dissolve polar and ionic solutes well, and nonpolar solvents dissolve nonpolar solutes
- Water is Polar! The indifferent Mickey Mouse…
- The oxygen will attract to positive ions and the hydrogens will attract to negative ions
- As temperature increases solubility of a solid will increase and solubility of a gas will decrease
- As pressure increases solubility of a gas will increase (CO2 in a soda can, high pressure)
- Solubility Table
- Left Side- Soluble (if an exception is present it will make it insoluble)
- Right Side- Insoluble (if an exception is present it will make it soluble)
- If an exception is present it will reverse the classification
- Group 1 metals (Na+, K+, Li+), NO3-, and NH4+ are always soluble…no exceptions
- Unsaturated- less solute dissolved than the maximum, more solute can still dissolve
- Saturated- maximum amount of solute is dissolved, no more solute can dissolve (can see solid undissolved at the bottom!)
- Supersaturated- more solute is dissolved than the solute can technically handle at that temperature (very unstable, have to heat up first and then cool slowly to create this type)
- Solubility Curves: each line shows the maximum number of grams of a substance that can be dissolved in 100g of water at various temperatures
- Hint: Look at temperature first and then match the line of the solute that is asked about
- When being asked to identify the type of solution, look up the temperature, the line of the solute, and the amount of grams dissolved, and then see if it is…Under the line- Unsaturated; On the line- Saturated; Over the line- Supersaturated
- Filtration- separates heterogeneous mixtures by filtering out larger solid particles
- Heterogeneous mixture- you can see the different substances (Ex. sand in water)
- Distillation- separates liquid homogeneous solutions by differences in boiling point
- Chromatography- separates homogeneous mixtures using differences in polarity
- Molarity- moles of solute / liters of solution
- Molality- moles of solute / kg of solvent
- Parts Per Million- (grams solute) / (grams solution) × 1,000,000
- Don’t forget to write “× 1,000,000”
- If given the initial amount of water instead of the initial amount of solution, then you have to add the solute and the solvent mass in the denominator
- Solute + Solvent = Solution
- Colligative Properties- properties of a solution that depend only on the number of solute particles dissolved
- As solute is dissolved in a solvent, the boiling point of that solvent will increase and the freezing point will decrease. (Alligator’s mouth!!! NOM NOM NOM HUNGRY FOR SOLUTE!! The more solute particles dissolved the wider the mouth opens- the more the BP increases and the FP decreases)
- Ionic solutes dissociate (separate) when they dissolve into their specific ions, when covalent just stays one particle…so usually ionic will change the BP or FP more than covalent
- Vapor pressure- the pressure that the vapor above a liquid causes due to gas particle collisons
- The more solute dissolved, the lower the vapor pressure will be
- Vapor Pressure vs temperature for various solvents
- Molecules with stronger intermolecular forces have lower vapor pressures
- The temperature at any specific vapor pressure represents the boiling point
- 101.3kPa represents the normal atmospheric pressure (1 atm)
- Dilution - M1V1 = M2V2
- M1 = initial concentration [M] or [mol/L]
- V1 = initial volume [L]
- M2 = final concentration [M] or [mol/L]
- V2 = final volume [L]
Unit 12 Highlights: Acids & Bases
- Acids
- Have sour taste
- Electrolytes (conduct electricity in aqueous solution because of mobile ions)
- React with some metals to form H2 gas (Zn + 2HCl → ZnCl2 + H2)
- Bases
- Have bitter taste
- Have slippery/soapy feeling
- Electrolytes (conduct electricity in aqueous solution because of mobile ions)
- Arrhenius Classification
- Acids release H+ ions in water
- Bases release OH- ions in water
- Bronsted-Lowry Classification
- Acids: Proton (H+) donors
- Bases: Proton (H+) acceptors
- Ex: HF + NH3 🡨🡪 F- + NH4+
- HF is the proton donor (it lost an H+)
- NH3 is the proton acceptor (it gained an H+)
- When H+ (a proton) is found in water it forms a hydronium ion: H+ + H2O → H3O+
- The more hydronium ions present the more acidic a solution is
- pH Scale ranges from 0-14
- 7 is neutral, below 7 is acidic, above 7 is basic
- Acids will have a lower pH (more H+), & bases will have a higher pH (less H+)
- Each number is a 10x difference in H+ concentration compared to the last
- pH 2 has 10x more H+ than 3
- pH 2 has 100x more H+ than 4
- pH 2 has 10x less H+ than 1
- pH 5 has 1/10th (0.1) as much H+ as 4
- pH 5 has 1/1000th (0.001) as much H+ as 2
- -log[H+]
- [H+] = 10-pH
- Indicators- change color based on pH
- Ex. Methyl orange (3.2 – 4.4, red to yellow) is red below 3.2, yellow above 4.4, and orange from 3.2-4.4
- Neutralization Reaction- Acid + Base → Salt + H2O
- (Ex. HBr + KOH → KBr + H2O)
- Salt- any ionic compound (Ex. NaCl, KBr, KCl, MgI2, etc.)
- Neutralization point is when moles of acid (H+) equals the moles of base (OH-)
- Titration- the process of adding measured volumes of an acid or base of known concentration to an acid or base of unknown concentration until neutralization occurs
- Used to find the concentration of the unknown acid or base
- MAVA = MBVB (Table T)
- Uses indicators to tell endpoint (when titration has reached neutralization around pH 7)
- Conjugate acid base pairs
- A conjugate base is what’s left after an acid donates a proton
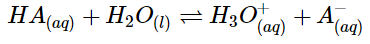
- A- is the conjugate base to HA
- H3O+ is the conjugate acid to H2O