CIE AS Biology 3.2: Rate of Reaction
The rate of reaction is the measure of how long it takes for a reaction to occur. In biochemical reactions, it is a measure of how long it takes for a reactant to be used up or for a product to be formed.
To measure the rate of reaction, we can measure:
- the amount of product formed or
- the rate of disappearance of a substrate using the complementary enzyme
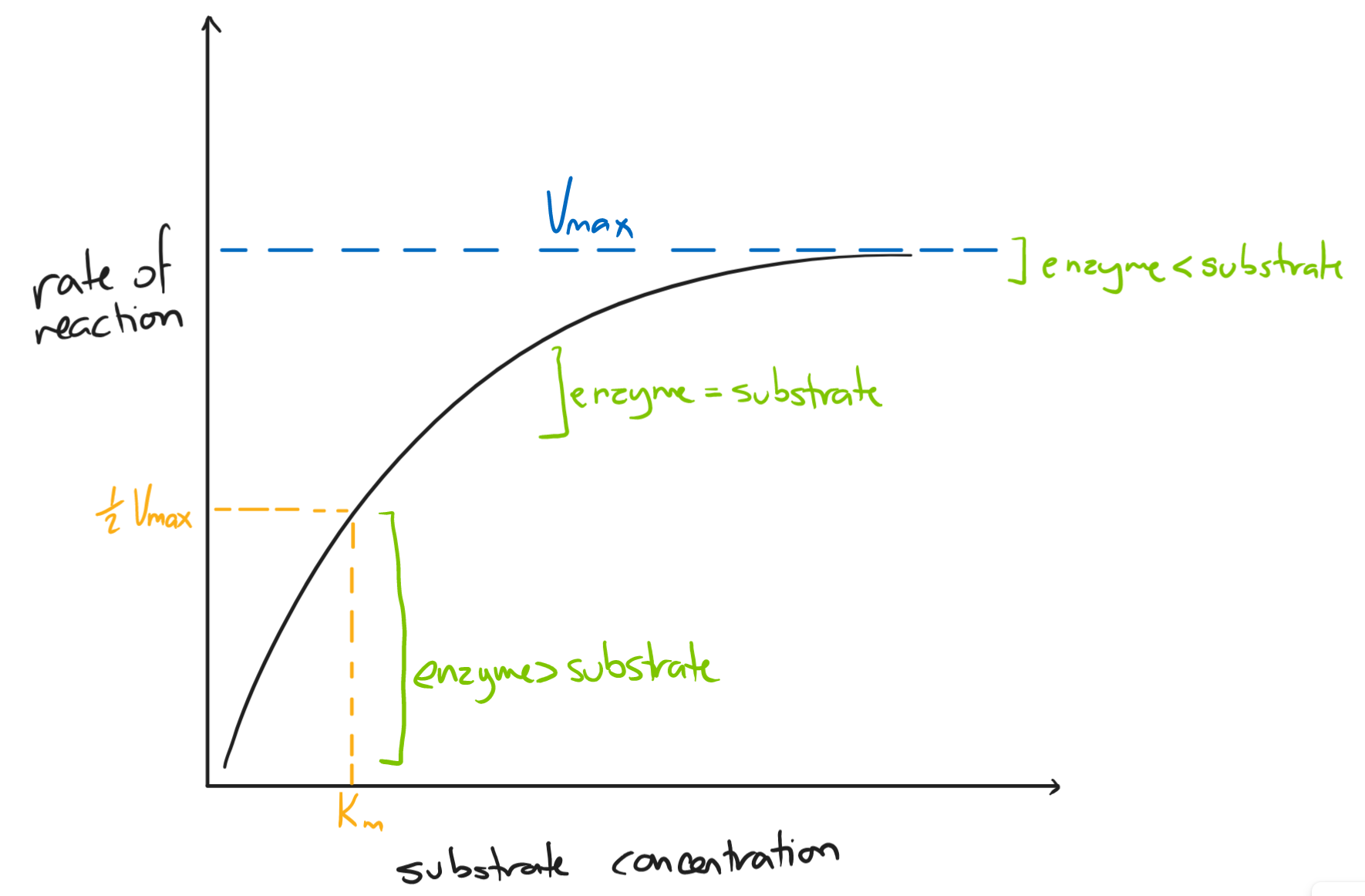
The initial rate of reaction is the fastest. As the reaction proceeds, more substrates bind to active sites, forming enzyme-substrate complexes and producing products.
Eventually, all the active sites are occupied and the concentration of enzymes becomes the limiting factor - this is where the graph plateaus and Vmax is calculated.
At half of Vmax, the Michaelis-Menten constant (Km) is calculated.
- the Michaelis-Menten constant (Vmax) is a measure of the affinity of an enzyme for a substrate during a reaction at half of its maximum capacity
- it is expressed as a concentration
- high Km → less affinity for its substrate; low Km → high affinity for its substrate
- affinity can vary depending on the substrate, temperature, pH, presence of particular ions, and presence of inhibitors
 Factors that Affect the Rate of Reaction
Factors that Affect the Rate of Reaction
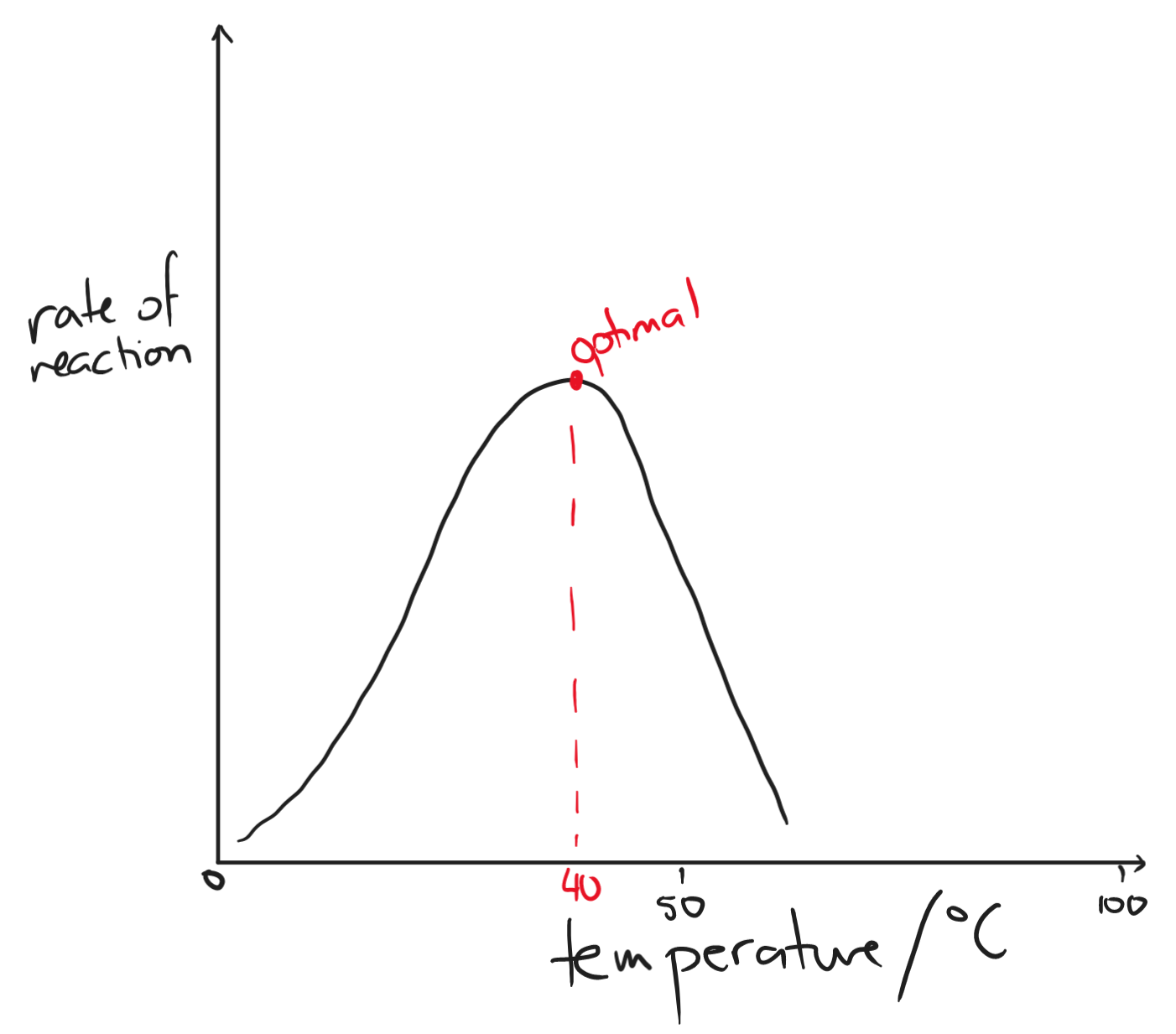
temperature
- as temperature increases, the rate of reaction increases
- due to collision theory, the substrates have more kinetic energy to collide with active sites with sufficient energy and with the right orientation
- temperature is too low → not enough collisions; temperature is too high → hydrogen bonds in the amino acid sequence are broken, enzyme denatures and loses shape
- optimal temperature is usually 30-40 degrees C
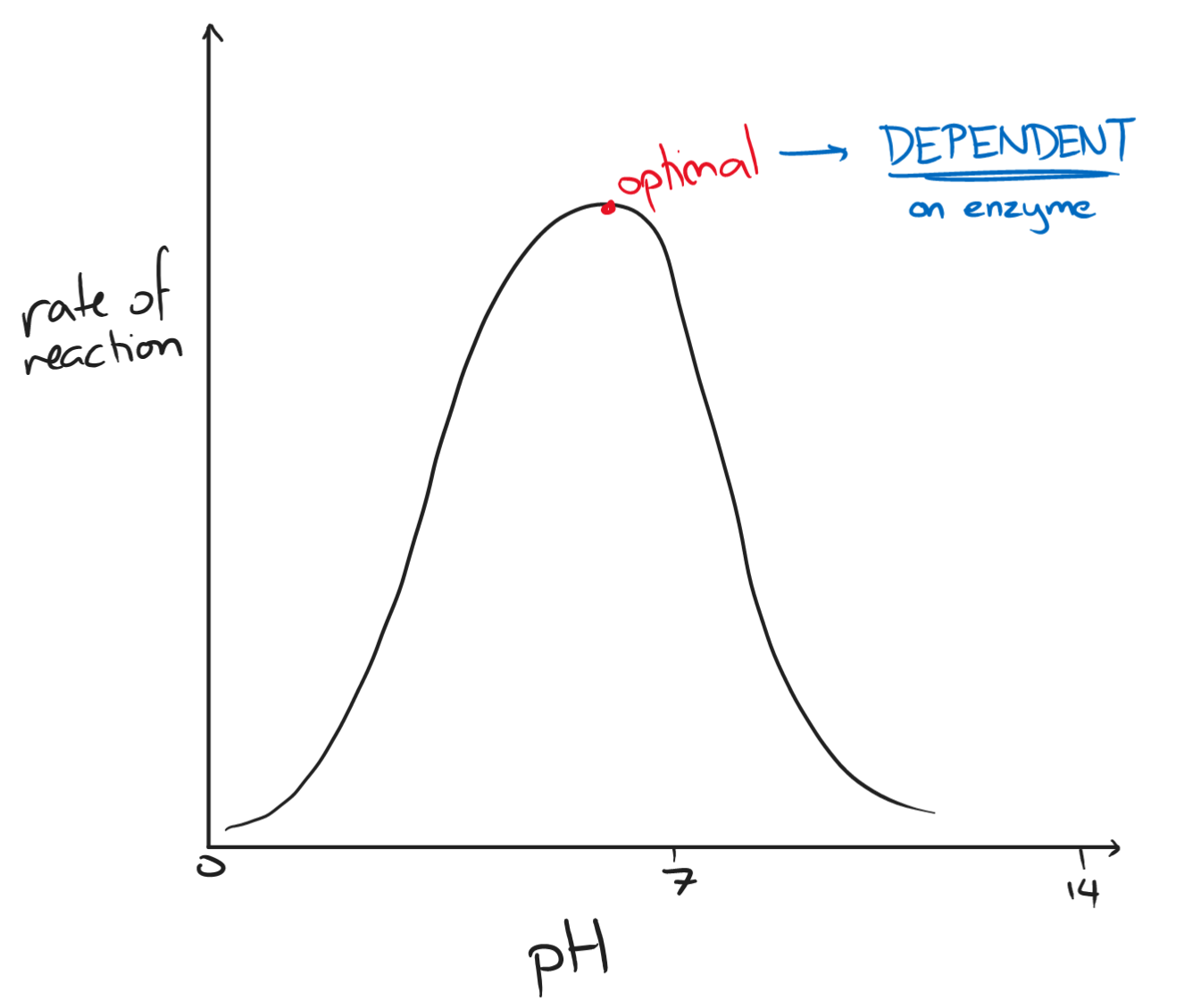
pH
- as pH increases, the rate of reaction increases
- too acidic/basic → ionic bonds holding the tertiary structure are disrupted, enzyme denatures
- optimal pH is dependent on each enzyme
- stomach enzymes have an optimal pH of around 1-2
- intestinal enzymes have an optimal pH of around 9 to 11
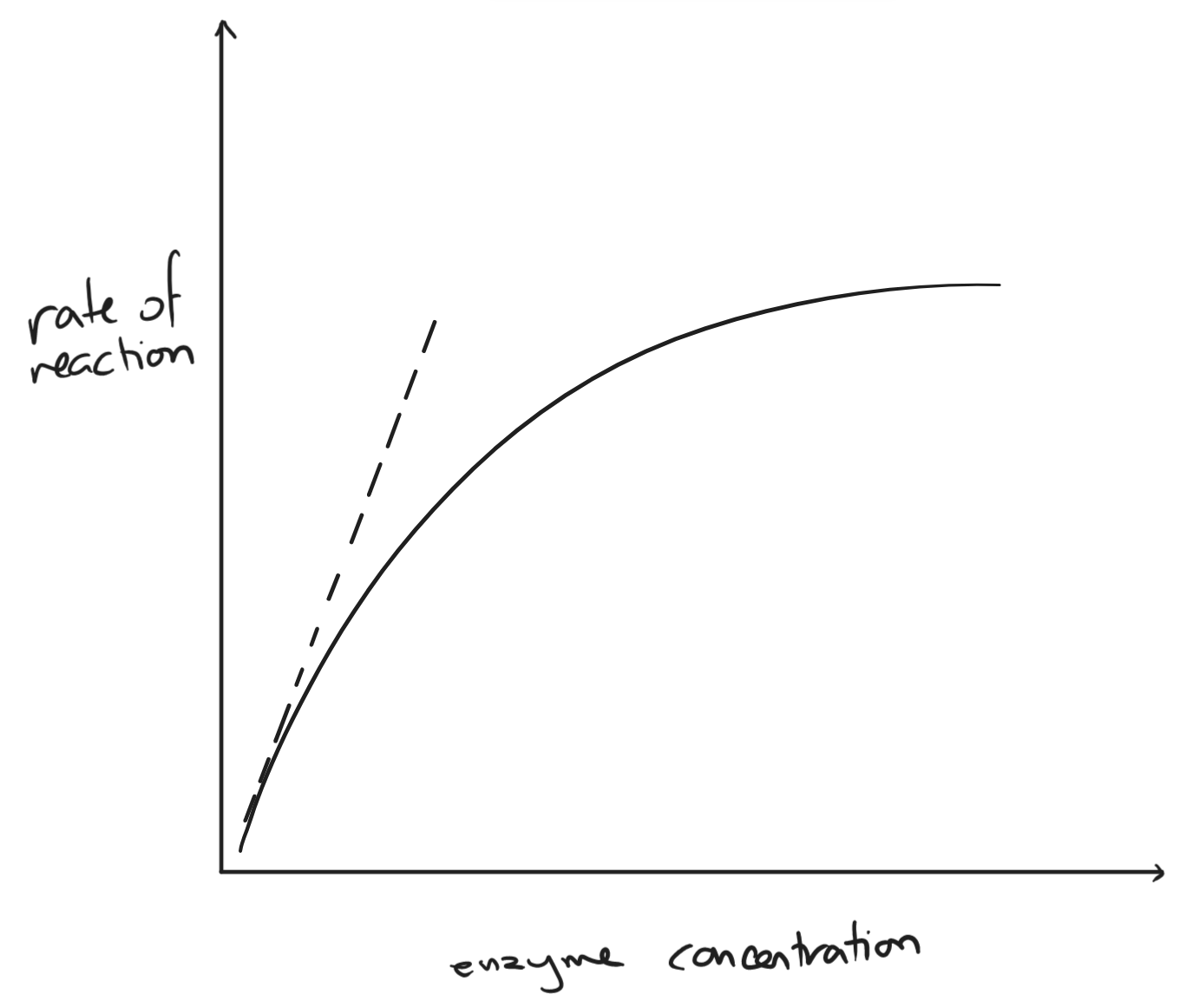
enzyme concentration
- as enzyme concentration increases, the rate of reaction increases
- slows down when substrate concentration becomes the limiting factor
- more enzymes than substrate → more empty active sites
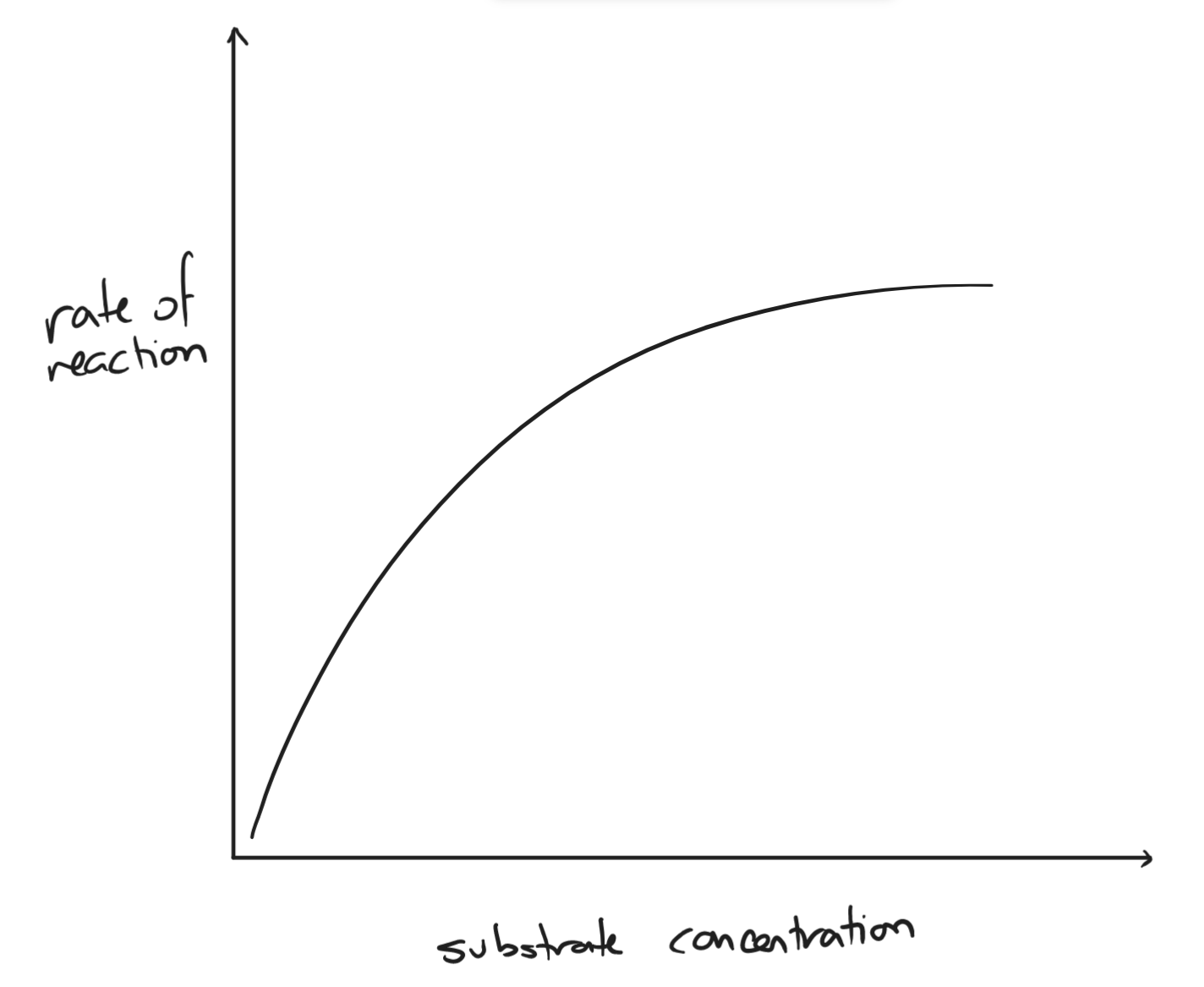
substrate concentration
- as substrate concentration increases, the rate of reaction increases
- slows down when enzyme concentration becomes the limiting factor
- more substrate than enzymes → all active sites are occupied
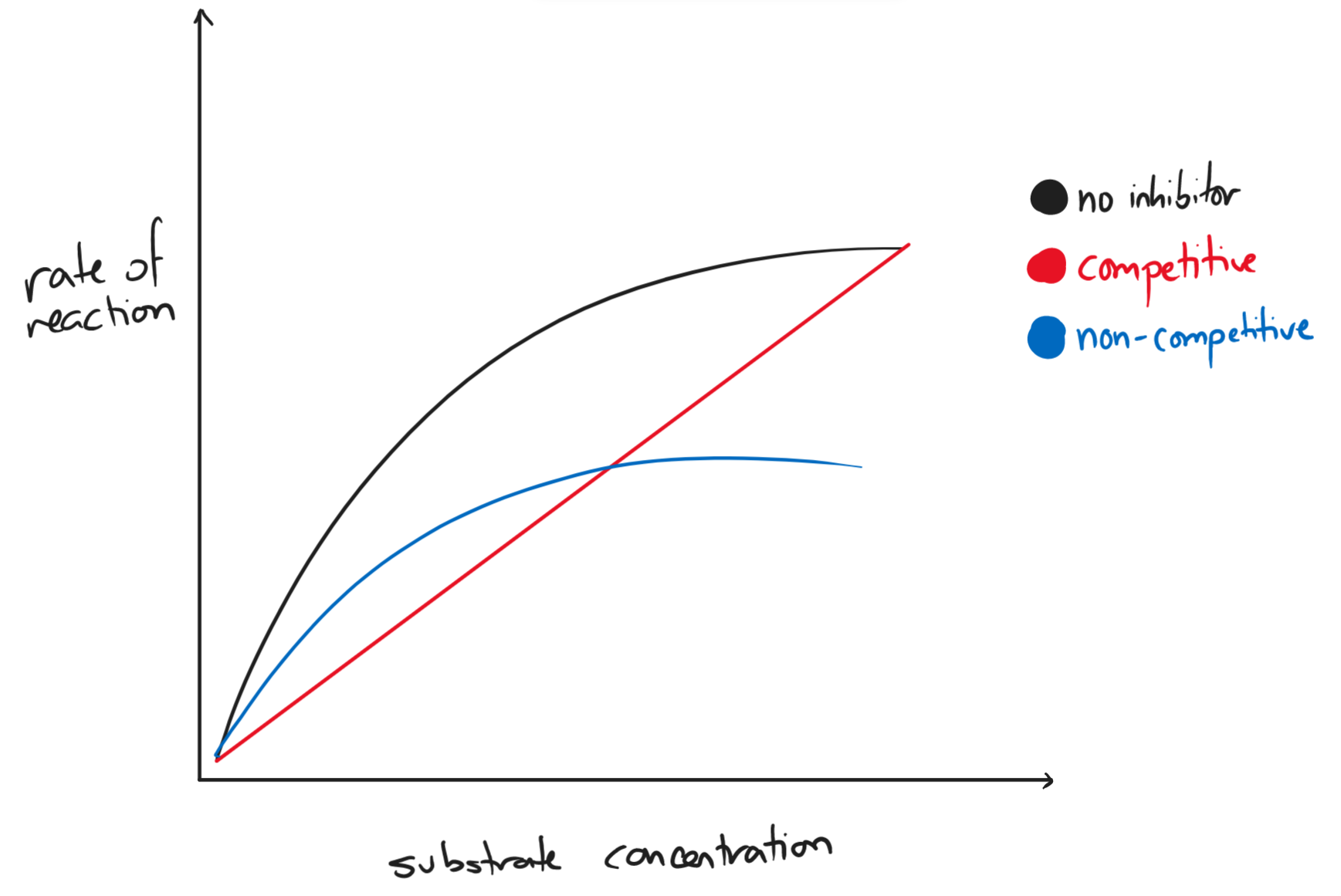
inhibitors
- competitive inhibitor: binds reversibly to the active site
- non-competitive inhibitor: binds to another part of the enzyme, altering the enzyme’s shape
- reversible inhibitors: binds to an enzyme to temporarily stop its processes
- used in metabolic reactions to act as regulators for product inhibition