Principles of Chemistry
1. States of matter
Definitions:
Solid: In solids, particles are held in fixed positions. This is because of the strong forces of attraction between particles in solids. Particles in a solid vibrate around their fixed positions
Liquid: In liquids, particles are randomly arranged and free to move past each other. The particles never stop moving. This is because of the weak forces of attraction between particles in liquids
Gas: In gases, particles are and free to move and far away from each other. This is because of the very weak forces of attraction between particles in gases. Particles are constantly moving.
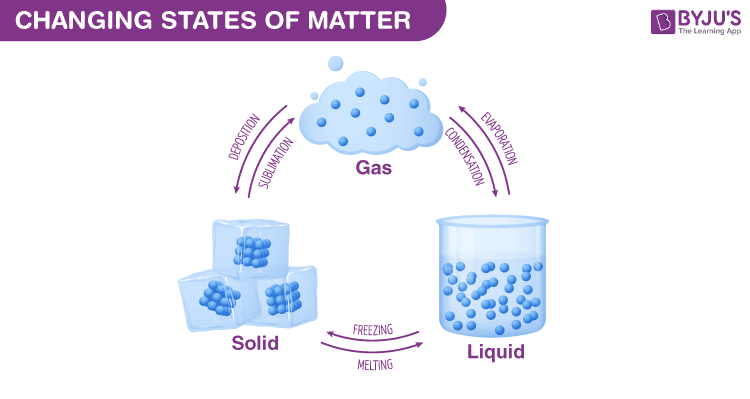
Changes of state:
Solids turn to liquids when they melt.
The increase in kinetic energy causes particles to vibrate more.
At a certain temperature, particles break free from their positions.
Liquids turn back into solids during freezing.
Liquids turn to gases when they evaporate.
Further increases in kinetic energy cause particles to move faster.
At a certain temperature, particles break their bonds.
Gases turn back into liquids during condensation.
Solids can turn directly into gases during sublimation.
If enough kinetic energy is supplied, substances can bypass the liquid phase.
Solutions and solubility
Definitions:
Diffusion: is the movement of particles from an area of high concentration to an area of lower concentration
Dilution: is lowering the concentration of a solute in a solution by simply adding more solvent to the solution
A solvent: is a liquid in which a solute dissolves
A solute: is the substance being dissolved - it is usually a solid
A solution: is the mixture formed when a solute dissolves in a solvent
Saturated: when no more solute can dissolve into a solution
Solubility: how many grams of a solute can dissolve in 100g of water
Things that affect solubility:
Temperature increases → solubility increases (solids)
Pressure increases → solubility increases (gasses)
The solvent
2. Element, compounds and mixtures
Definitions + properties:
Elements: consist of only one type of atom.
For example, hydrogen (H2), nitrogen (N2) and carbon (C) are all elements.
Compounds: are made up of two or more different elements.
These elements are chemically bonded to one another.
For example carbon (C) and oxygen (O2) can react with each other to form carbon dioxide (CO2).
The properties of compounds are often very different to their original elements
Mixtures: are a combination of two or more different elements.
Mixtures are not chemically bonded to one another.
Instead they are physically in close proximity.
For example, sand and water can form a mixture.
Mixtures will show the properties of both elements
Pure substances: are made out of only one element or compound
Pure substances have melting points and boiling points that will not change
Methods of Separation:
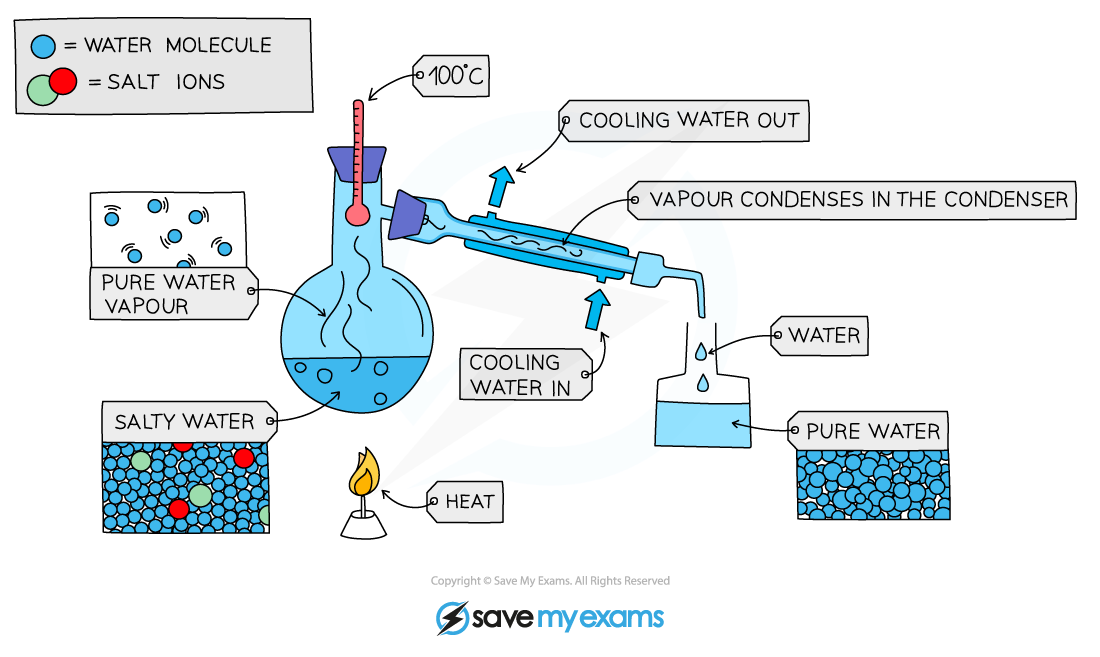
Distillation: is used to separate out solutions
Simple distillation: only works when the components have very different boiling points
1. The solution is heated.
The part of the solution with the lowest boiling point will evaporate.
The vapor is cooled and condensed.
This is done with the aid of a cooling water jacket.
The newly condensed, pure liquid is collected in a new container
The rest of the solution remains in the flask.
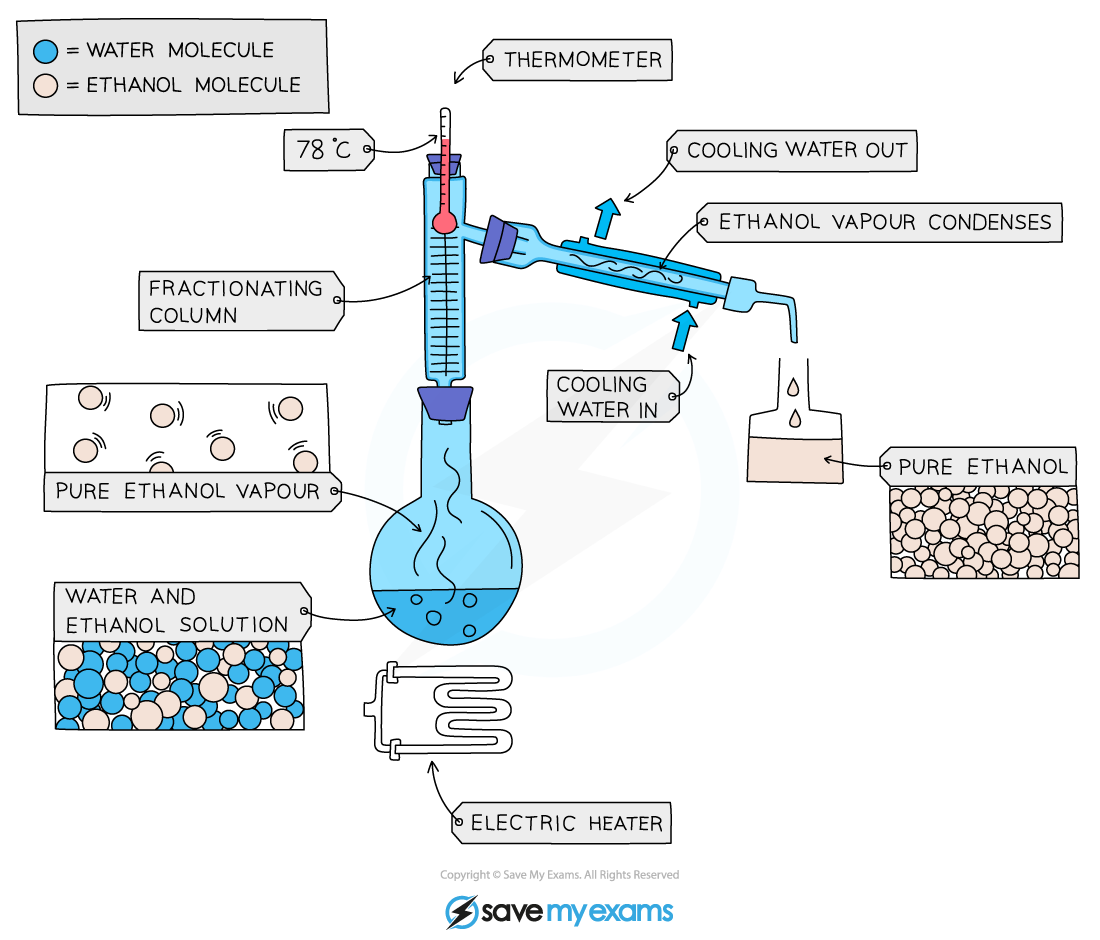
Fractional distillation: used to separate mixtures of liquids
These liquids will have different boiling points.
The liquid with the lowest boiling point will evaporate first.
The evaporating liquid will rise up a fractionating column.
It will then be carried to a condenser.
Here it will reform as a liquid.
The temperature is then raised so the second liquid can be collected.
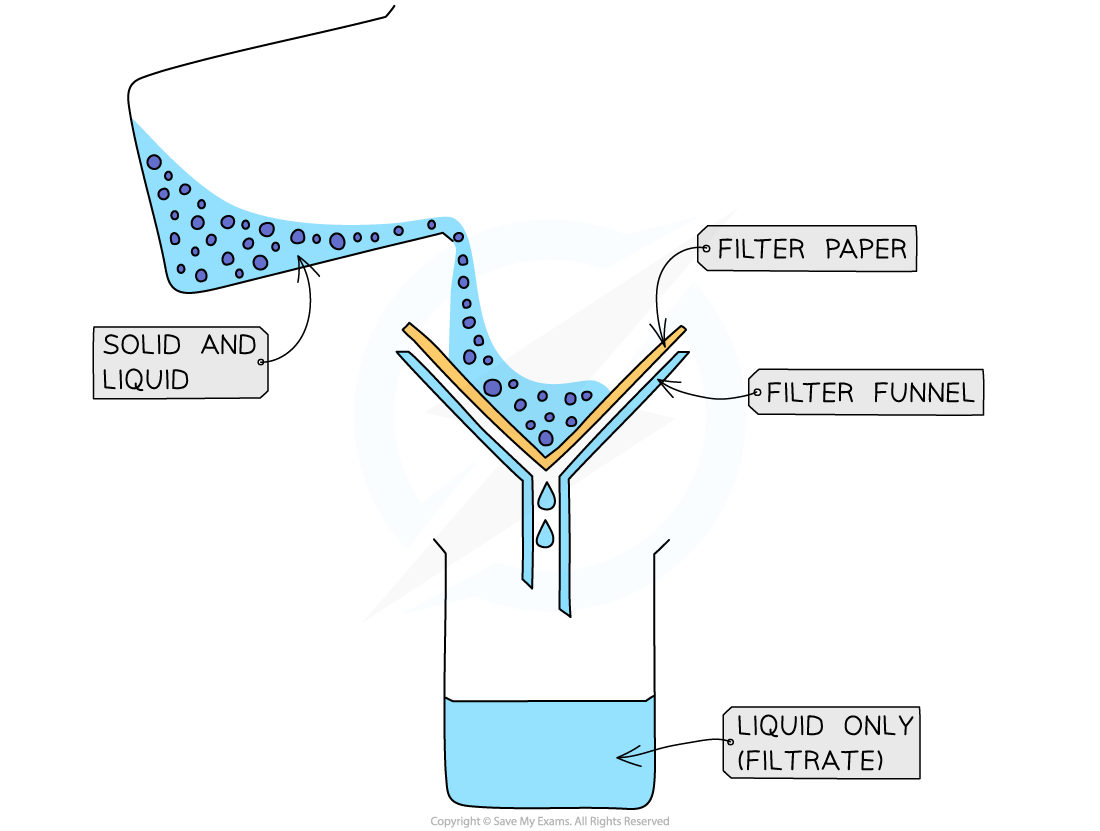
Filtration: used to separate insoluble solid from a liquid
The filter paper is placed in a funnel above a beaker.
The mixture is poured into the funnel.
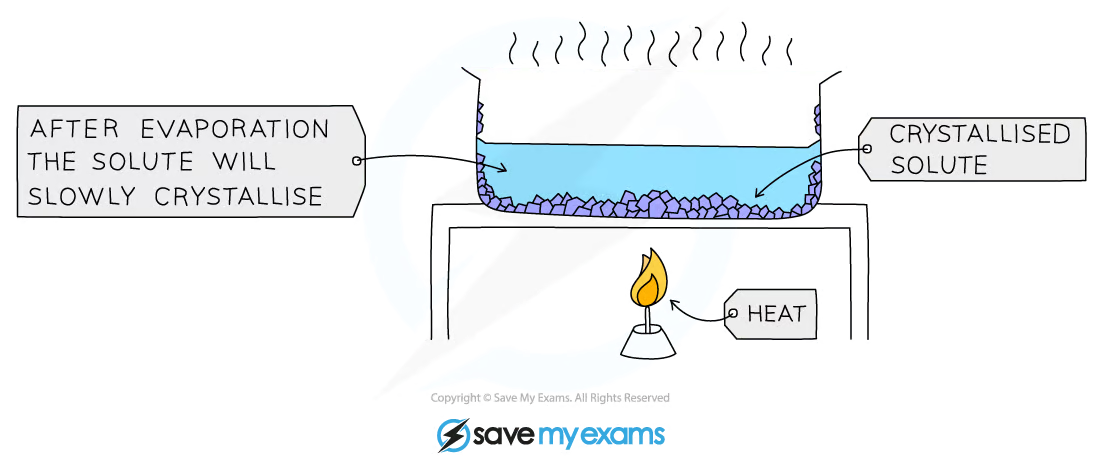
Crystallization: separates soluble solids from a solution
The solution is heated in an evaporating dish.
Some of the liquid evaporates, creating a more concentrated solution
As the solution cools, crystals of solute begin to form
This is because the solute is much less soluble when it is cold.
The crystals are then filtrated from the solution and dried
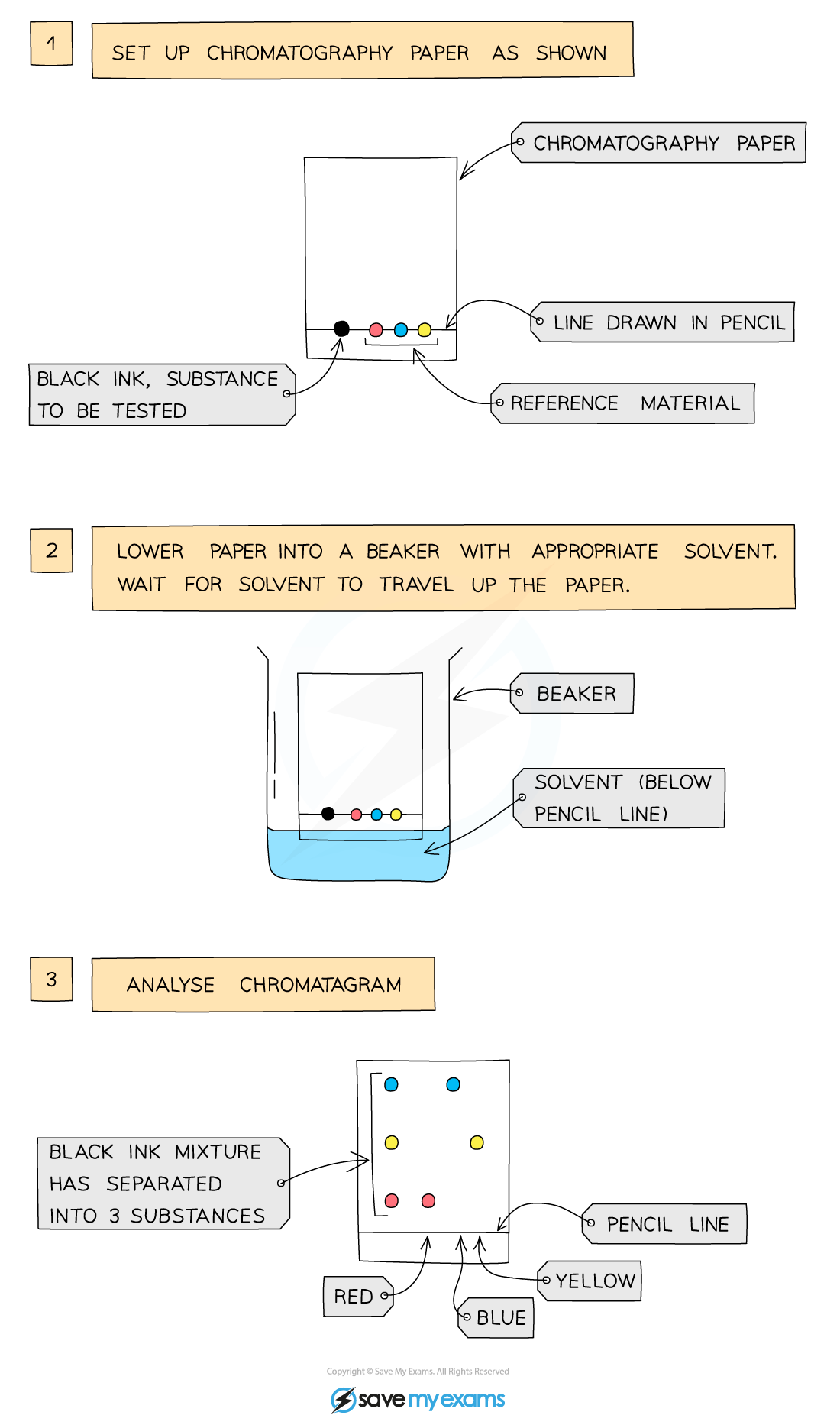
Paper chromatography: is a way to separate mixtures
The mixtures are separated by their solubility in that particular solvent
A pencil line is drawn close to the bottom of the filter paper
The pencil is insoluble so it won’t dissolve in the solvent
Spots of different inks are placed equidistant from each other on the pencil line
The paper is lowered into the solvent but it should not touch the inks (below the pencil line the solvent should be)
As the solvent goes up the filter paper, it carries the dye with it
3. Atomic structure
Definitions:
An atom is the smallest particle of an element.
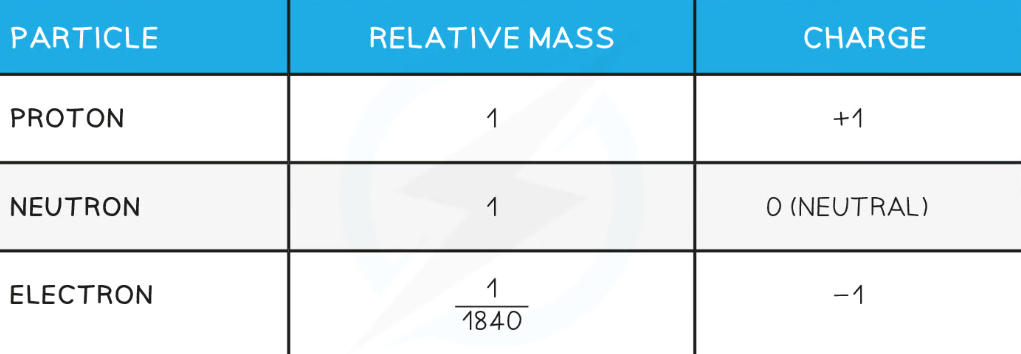
An atom contains three subatomic particles:
Protons - these are heavy and positively charged.
Neutrons - these are heavy and neutral.
Electrons - these have hardly any mass and are negatively charged
Structure of an atom:
The protons and the neutrons are found in the nucleus
The electrons are found on the other shells of the atom
Atoms V.S. Molecules

Terms:
Atomic number: number of protons
Mass number: number of protons + number of neutrons
Isotopes: two (or more) atoms of the same type that have different mass numbers (same number of protons but different neutrons number)
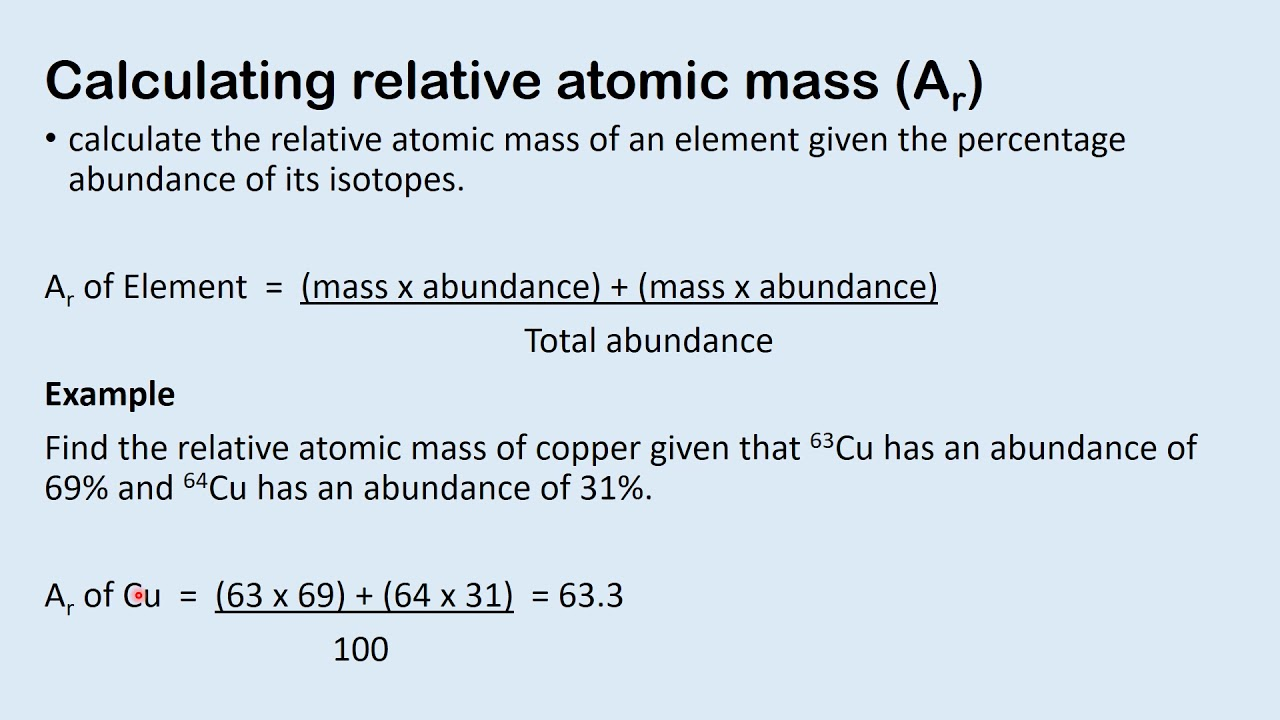
Relative atomic mass: The average mass of naturally occurring atoms of an element on a scale where the carbon 12 atom has a mass of exactly 12 units. Basically since atoms have such a small mass, the atoms mass is compared to the ratio between carbon 12 where its relative atomic mass is 12
How to calculate the relative atomic mass of an element:
4. The periodic table
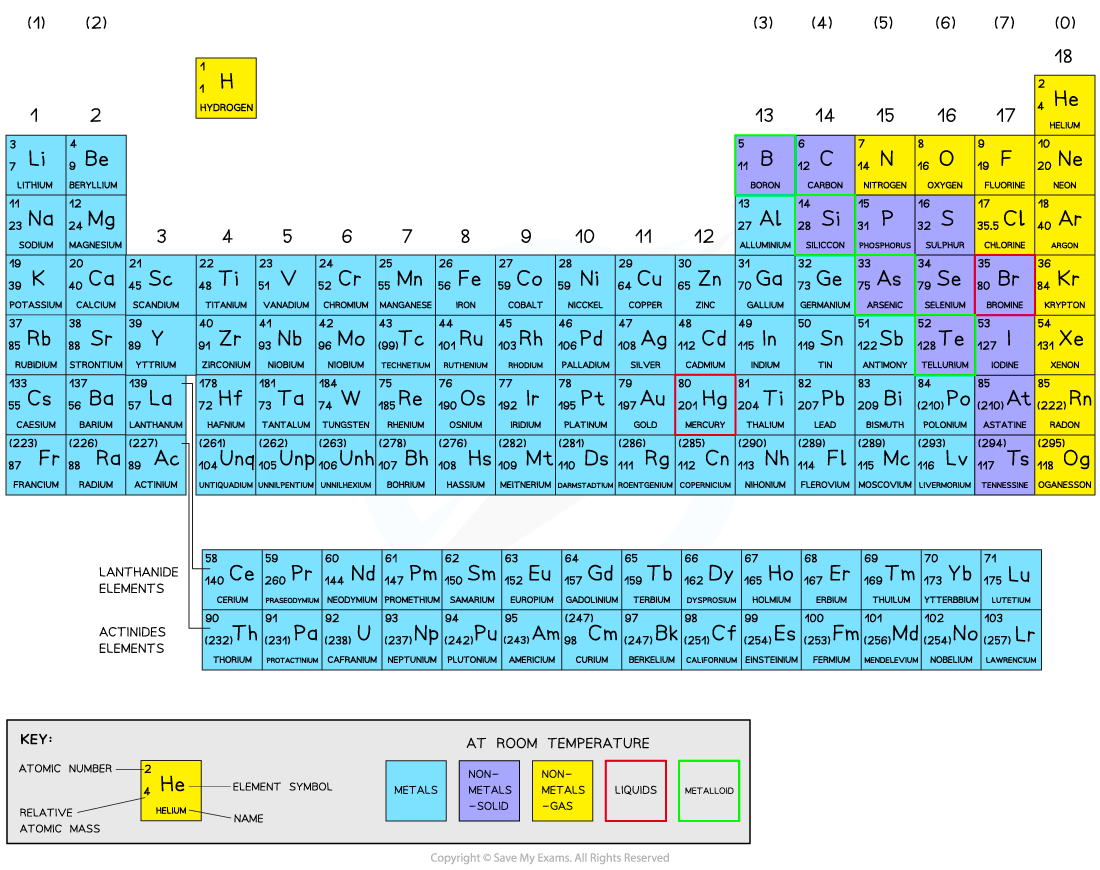
Elements are arranged in order of atomic (proton) number
Groups: These are the vertical columns that show how many outer electrons each atom has and are numbered from 1 – 7, with a final group called group 0 (instead of group 8)
Periods: These are the horizontal rows that show the number of shells of electrons an atom has and are numbered from 1 - 7
What is the electron configuration of an atom?
The electronic configuration of an element tells you how many electrons are in each shell around an electron’s nucleus
e.g. sodium has 11 electrons: 2 in its most inner shell, then 8, then 1 in its outermost shell
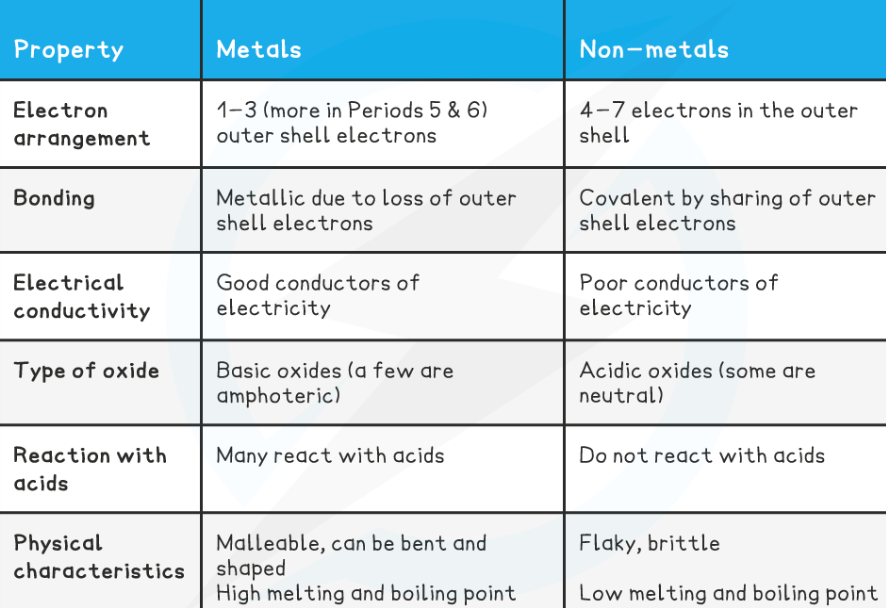
Metals and non-metals in the periodic table:
Metals = elements that react to form positive ions.
Majority of elements are metals.
Found to the left and towards the bottom of the periodic table.
Non-metals = elements that do not form positive ions.
Found towards the right and top of the periodic table
Why doesn’t group 0 react?
They are unreactive and do not easily form molecules, because they have a stable arrangement of electrons (always have full outer shells)
5. Chemical formulae
1. Calculating relative mass
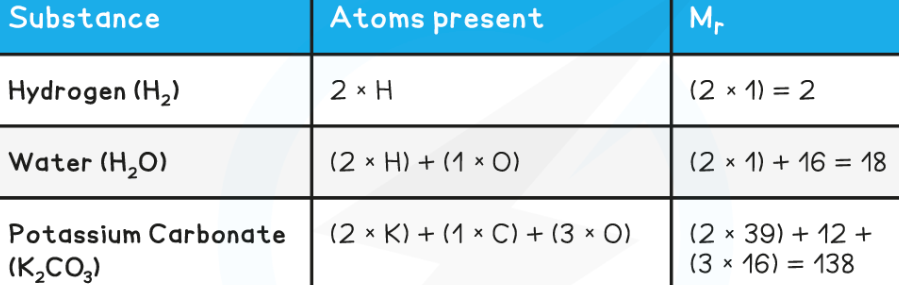
What is Mr (Relative Molecular Mass) in chemistry?
We have seen previously that the symbol for the relative atomic mass is Ar
This is calculated from the mass number and relative abundances of all the isotopes of a particular element
To calculate the Mr of a substance, you have to add up the relative atomic masses of all the atoms present in the formula
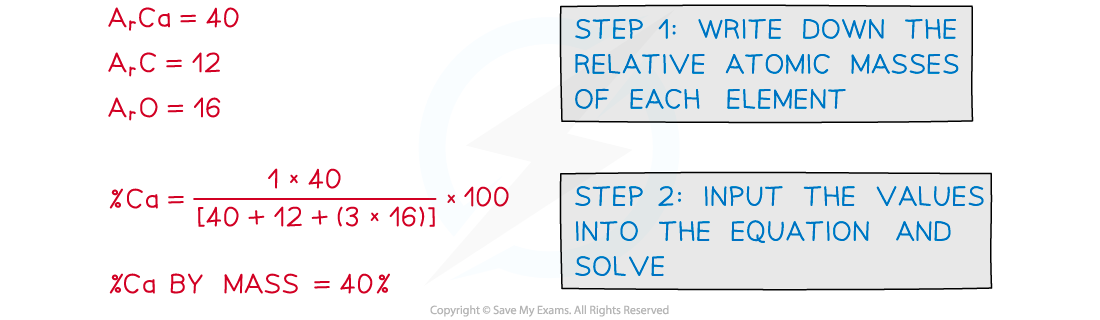
Calculating percentage by mass of an element in a compound:
Example:
2. Calculating Moles, Mass & RFM
The Mole:
Chemical amounts are measured in moles
The symbol for the unit mole is mol
One mole of a substance contains the same number of the stated particles, atoms, molecules, or ions as one mole of any other substance
Molar mass: One mole of any element is equal to the relative atomic mass of that element in grams or for a compound the relative formula mass in grams
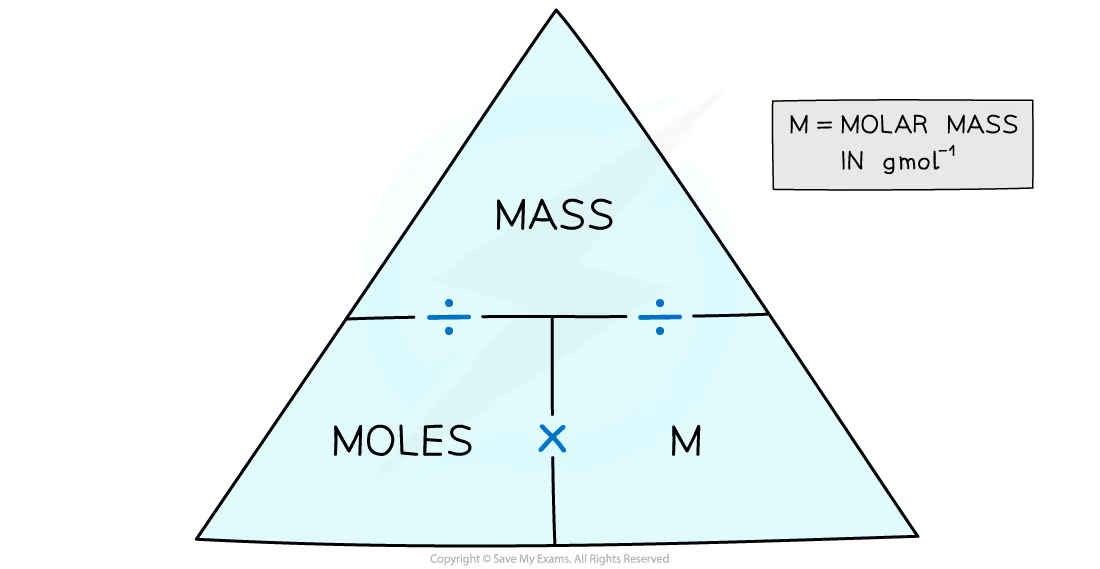
Example: How many moles are in 2.64 g of sucrose, C12H22O11 (Mr = 342.3)?
Answer:
The number of moles is found by mass ÷ molar mass
This comes to 2.64 g ÷ 342.3 g mol-1 = 7.71 x 10-3 mol
3. Calculating reactive masses
The information given in the question is used to find the amount in moles of the substances being considered
Then, the ratio between the substances is identified using the balanced chemical equation
Once the moles have been determined they can then be converted into grams using the relative atomic or relative formula masses
Example:
Calculate the mass of magnesium oxide that can be made by completely burning 6.0 g of magnesium in oxygen in the following reaction:
Balancing Equations using Reacting Masses
If the masses of reactants and products of a reaction are known then we can use them to write a balanced equation for that reaction
This is done by converting the masses to moles and simplifying to find the molar ratios
Example: A student reacts 1.2 g of carbon with 16.2 g of zinc oxide. The resulting products are 4.4 g of carbon dioxide and 13 g of zinc. Determine the balanced equation for the reaction.

4. Calculating percentage yield
Yield is the term used to describe the amount of product you get from a reaction
Example: Copper(II) sulfate may be prepared by the reaction of dilute sulfuric acid with copper(II) oxide. A student prepared 1.6 g of dry copper(II) sulfate crystals.
Calculate the percentage yield if the theoretical yield is 2.0 g.
Actual yield of copper(II) sulfate = 1.6 g
Percentage yield of copper(II) sulfate = 1.6/2.0 × 100 = 80%
5. Empirical & Molecular Formulae
Empirical formula: gives the simplest whole number ratio of atoms of each element in the compound
e.g.
6. Calculate Concentrations of Solutions
Example: Calculate the amount of solute, in moles, present in 2.5 dm3 of a solution whose concentration is 0.2 mol dm-3.
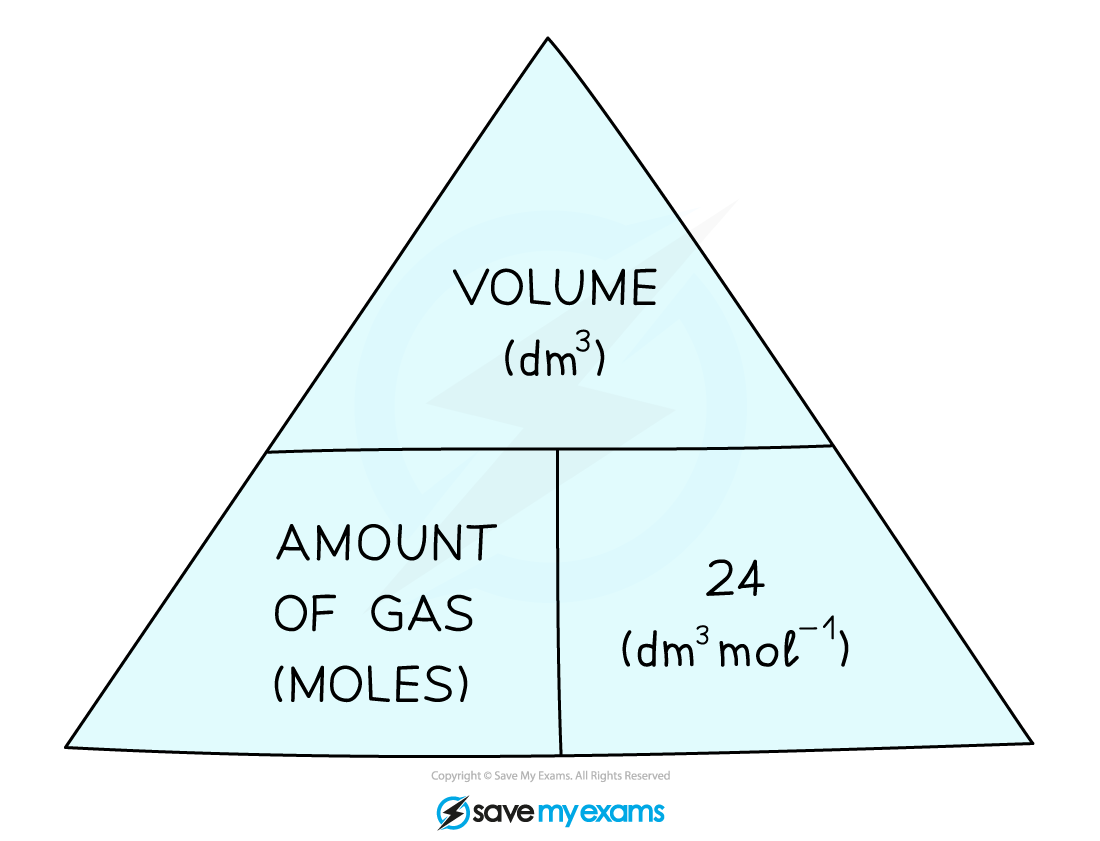
7. Calculate Volumes of Gases
At room temperature and pressure, the volume occupied by one mole of any gas was found to be 24 dm3 or 24,000 cm3
This is known as the molar gas volume at RTP (room temperature and pressure)
6. Ionic bonding
Definition: Ions are atoms that have lost or gained electrons
Metal atoms lose electrons to become positively charged ions
Nonmetal atoms gain electrons to become negatively charged ions
Terms:
Cation: positive ion
Anion: negative ion
Definition: Ionic compounds form when metals give one or more electrons to non-metals.
The strong attraction between a cation and an anion is called an electrostatic attraction. The forces act in all directions in the lattice.
Properties of ionic bonding:
Ionic compounds have high melting and boiling points.
Ionic compounds are hard and brittle.
Ionic compounds dissociate into ions when dissolved in water.
Solutions of ionic compounds and melted ionic compounds conduct electricity, but solid materials do not.
Reason for these properties:
There are strong electrostatic forces of attraction between oppositely charged ions
Requires a lot of energy to overcome these forces of attraction
7. Covalent bonding
Definition: Covalent bonding occurs in most non-metallic elements and in compounds of nonmetals
How the bonding works:
Strong bonds between atoms that are covalently bonded are the result of electrostatic attraction between the positive nuclei of the atoms and the pairs of negative electrons that are shared between them.
Simple molecular structures
They are are attracted to each other through intermolecular forces
Substances with a simple molecular structures are gases or liquids, or solids with low melting and boiling points
This is because even if the covalent bonds are strong, between the compounds the intermolecular forces are weak
Giant covalent structures
Substances that consist of giant covalent structures are solids with very high
melting and boiling points.
All of the atoms in these structures are linked to other atoms by strong covalent
bonds
Examples:
Diamond
very hard
doesn’t conduct electricity
Graphite
soft
conducts electricity
Properties:
do not conduct electricity (except graphite)
8. Metallic bonding
Metals consist of giant structures of atoms arranged in a regular pattern
The electrons in the outer shell of metal atoms are delocalized and so are free to move through the whole structure.
The sharing of delocalized electrons makes a strong electrostatic attraction between negatively charged electrons and positive metal ions
Properties:
Most metals have high melting and boiling points.
They can conduct heat and electricity because of the delocalized electrons in their structures.
The layers of atoms in metals are able to slide over each other, so metals can be bent and shaped
9. Electrolysis
When an electric current is passed through a molten ionic compound the compound decomposes or breaks down
The process also occurs for aqueous solutions of ionic compounds
Covalent compounds cannot conduct electricity hence they do not undergo electrolysis
Ionic compounds in the solid state cannot conduct electricity either since they have no free ions that can move and carry the charge
Key terms:
Electrode: is a rod of metal or graphite through which an electric current flows into or out of an
electrolyte
Electrolyte: is the ionic compound in molten or dissolved solution that conducts the electricity
Anode: is the positive electrode of an electrolysis cell
Anion: is a negatively charged ion which is attracted to the anode
Cathode: is the negative electrode of an electrolysis cell
Cation: is a positively charged ion which is attracted to the cathode
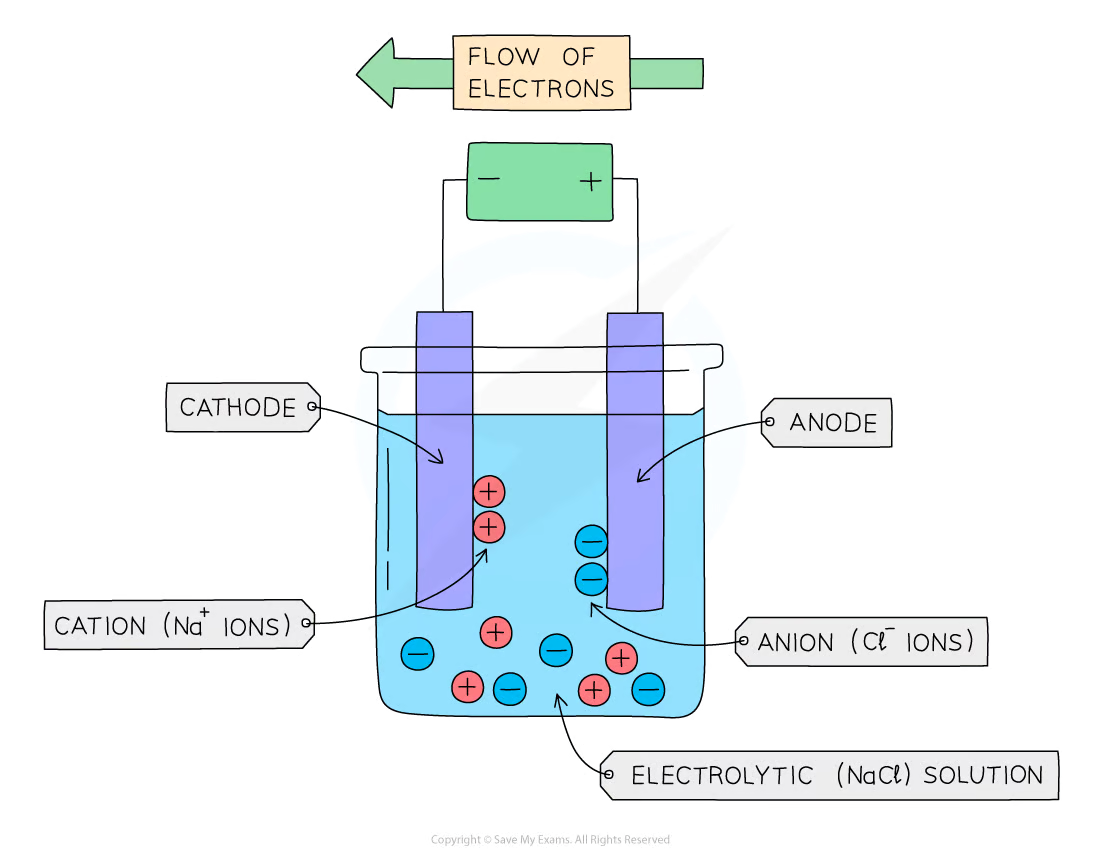
Positive electrode (anode)
Non-metal ions (other than hydrogen) are attracted to the positive electrode
Non-metal ions will lose electrons to form the non-metal
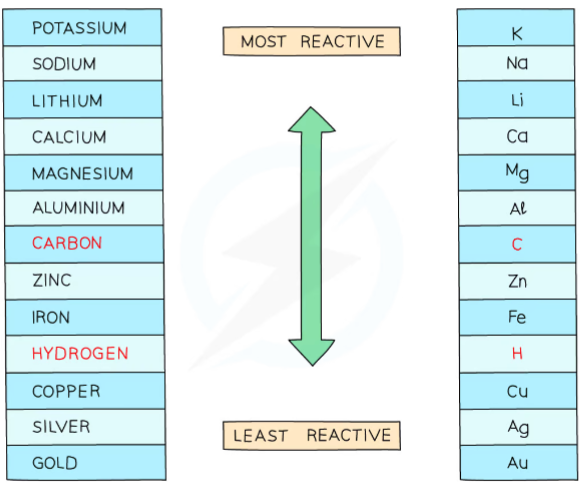
The product formed depends on which ion loses electrons more readily, with the more reactive ion remaining in solution
Negative electrode (cathode)
Hydrogen and metal ions attracted to the negative electrode but only one will gain electrons
Either hydrogen or metal will be produced
If the metal is above hydrogen in reactivity series, then hydrogen will be produced and bubbling will be seen at the cathode
Electrolysis of Aqueous Solutions
Aqueous solutions will always have water present (H2O)
Which ions get discharged and at which electrode depends on the relative reactivity of the
elements involved
Anode:
If halide ions (Cl- , Br- , I- ) and OH- are present then the halide ion is discharged at the anode, loses electrons and forms a halogen (chlorine, bromine or iodine)
If no halide ions are present, then OH is discharged at the anode, loses electrons and forms
oxygen gas
Cathode:
If the metal is above hydrogen in the reactivity series, then hydrogen will be produced and bubbling will be seen at the cathode
This is because the more reactive ions will remain in the solution, causing the least reactive ion to be discharged
Gas tests:
Hydrogen: squeaky pop test
The test for hydrogen consists of holding a burning splint held at the open end of a test tube of gas.
If the gas is hydrogen it burns with a loud “squeaky pop” which is the result of the rapid combustion of hydrogen with oxygen to produce water.
Chlorine: Litmus paper
Chlorine is an acidic gas that also acts as a bleach. Damp
litmus paper is bleached white when it is placed in chlorine.
If damp blue litmus paper is used, the paper turns red then white.
Carbon dioxide: Limewater
Carbon dioxide reacts with calcium hydroxide solution to produce a white precipitate of calcium carbonate.
Limewater is a solution of calcium hydroxide. If carbon dioxide is bubbled through limewater, the limewater turns milky or cloudy white.
Oxygen: glowing splint relights
Oxygen supports combustion. If oxygen is present in a test tube, a glowing splint relights when it is held inside.
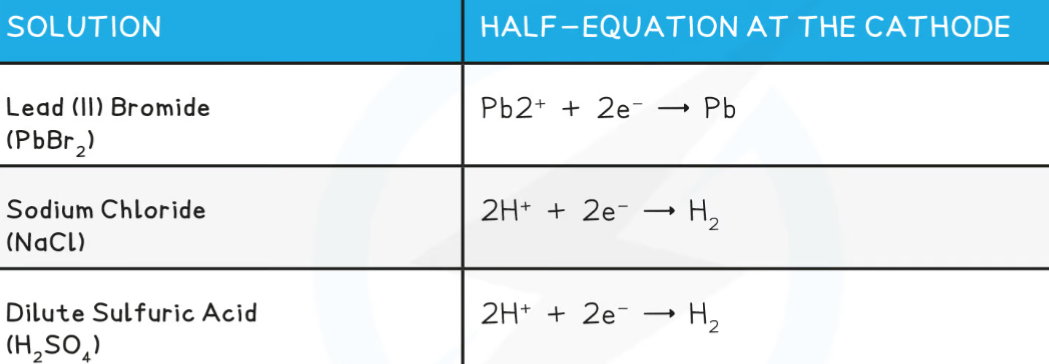
Reactions at the Electrodes & Ionic Half-Equations:
Oxidation is when a substance loses electrons
Reduction is when a substance gains electrons
At the anode, negatively charged ions lose electrons and are thus oxidized
At the cathode, the positively charged ions gain electrons and are thus reduced
Half equations show the oxidation and reduction of the ions involved
Transfer of Charge
During electrolysis the electrons move from the power supply towards the cathode
Positive ions within the electrolyte move towards the negatively charged electrode which is the cathode
Here they accept electrons from the cathode and either a metal or hydrogen gas is produced
Negative ions within the electrolyte move towards the positively charged electrode which is the anode
If the anode is inert (such as graphite or platinum), the ions lose electrons to the anode and form a nonmetal or oxygen gas
If the anode is a reactive metal, then the metal atoms of the anode lose electrons and go into solution as ions, thinning the anode
Electroplating: is a process where the surface of one metal is coated with a layer of a different metal
The anode is made from the pure metal you want to coat your object with
The cathode is the object to be electroplated
Uses of electroplating
Electroplating is done to make metals more resistant to corrosion or damage
e.g, chromium and nickel plating
It is also done to improve the appearance of metals,
e.g. coating cutlery and jewelry with silver
Conductors:
Solids such as metals or graphite
Liquids such as molten lead bromide or molten metals
Solutions such as sodium chloride solution
Insulators: Most insulators are solids of plastic, rubber or ceramic