Unit 4.3.1: Electrode Potentials
3.1.11
Electrochemical cells
Electrochemical cells can be made from two different metals dipped in salt solutions of their own ions (i.e. Fe (s) in Fe2+ (aq) ) and connected by a wire.
There are always two reactions going on: Oxidation and Reduction - making this a redox process
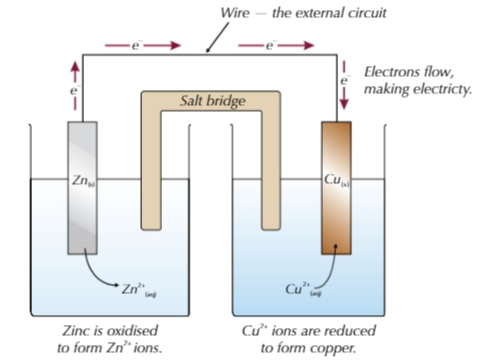
- The metal electrodes below are dipped in a s olution of their respective ions, connected via a salt bridge.
- Zinc loses electrons more easily than copper so is by convention placed on the left.
- This also means that the Zn electrode is more positive than the Cu electrode meaning the Zn(s) is oxidised to Zn2+, the electrons travel to the Cu2+ ions and reduces them to Cu(s)
- The salt bridge allows ions to flow between the half-cells and balance the charges, completeing the circuit.
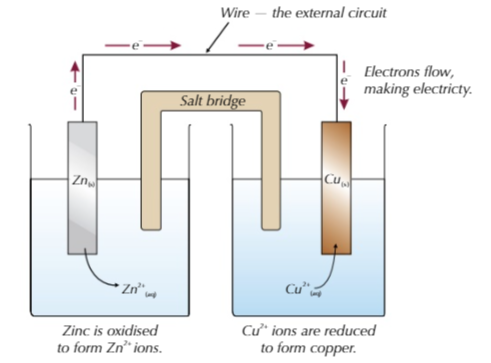
Electrons flow through the wire from the most reactive to the least reactive.
Cell Potential/Electromotive Force (EMF) - The voltage between two half-cells
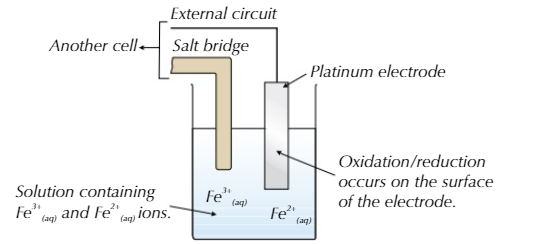
You can also have a half-cell involving solutions of 2 aqueous ions of the same element
- A platinum electrode is used because it is inert (wont react)
- The conversion from Fe2+ to Fe3+ happens on the surface of the electrode
 Electrode Potentials
Electrode Potentials
The reactions that occur at each electrode in a cell are reversible
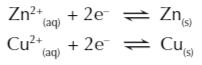
The direction the reaction goes in depends on how easily each metal is oxidised
This is measured using electrode potential
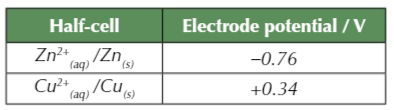
More negative electrode potential (V) = more easily oxidised
- Zinc has a more -ve electrode potanital so is oxidised
- Copper has a more +ve electrode potantial so is reduced
 Drawing Electrochemical Cells
Drawing Electrochemical Cells
Theres a shorthand way to drawing those big electrochemical cells:
- | = state separation, you put this between substances of different phases in the same half-cell
- || = salt bridge, you use this to separate the two half-cells
Therefore, this electrochemical cell can be written as
Zn(s) | Zn2+ (aq) || Cu2+ (aq) | Cu (s)
By convention, unless it is Hydrogen, you always put the most negative electrode on the left.
Follow these steps in order to draw electrochemical cells in shorthand:
- Use the electrode potentials to work out which haldf-cell goes on the left and which goes on the right (unless you have Hydrogen, which always goes on the left)
- Write out the left-hand half-equation as an oxidation reaction, and the right-hand half-equation as a reduction equation
- Write out the reactants and products of the oxidation reaction followed by the reactants and products of the reduction reaction
- Add in a salt bridge with || between the oxidation and reduction reactions
- Add in your phase separator, | , between any reagents that have different phases, and a comma between any of the same phase.
- If the element and electrode are separate, put the electrode on the outside and separate using a |
Calculating the cell potential
The cell potential will always be a positive voltage because the more negative value is being subtracted from the more positive value
