Week 2 part 1: Membranes- transport and potentials
Cell and ion gradients
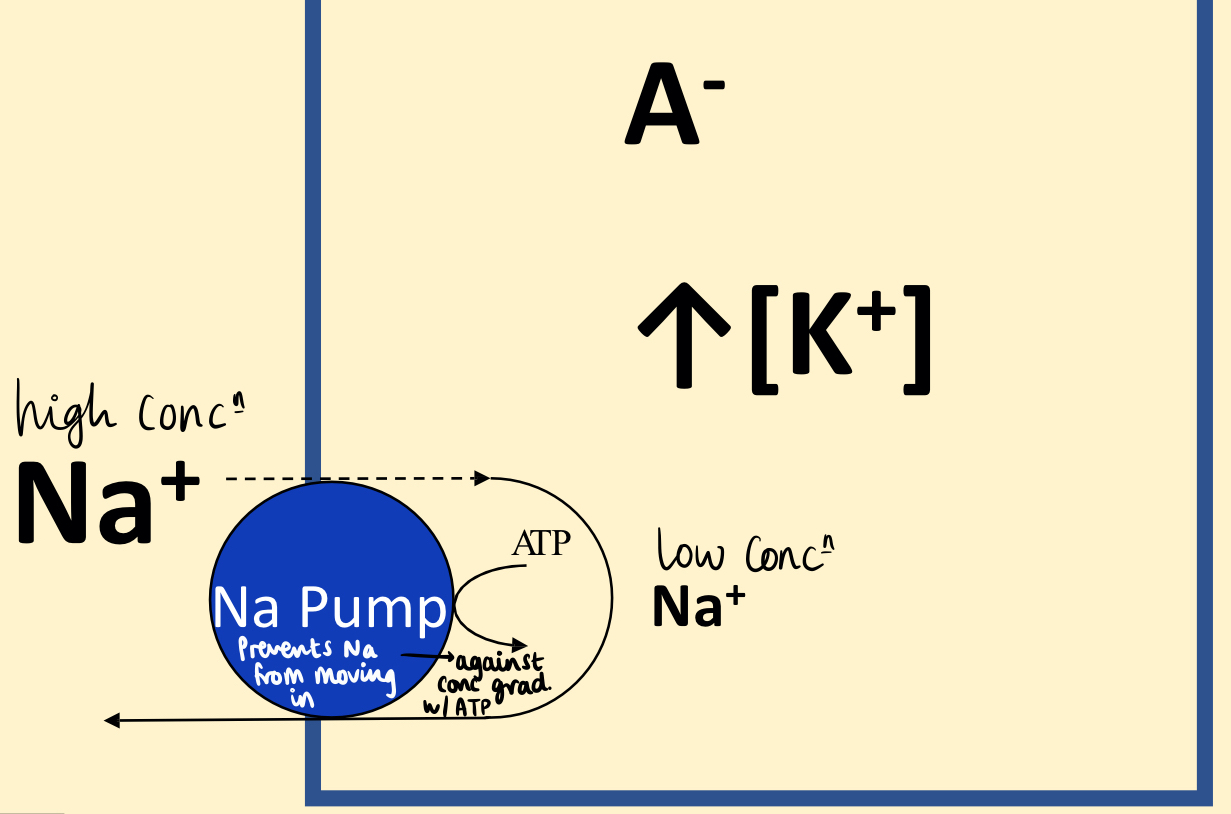
All intracellular values (K+, A-, Cl- ) are higher (outside) than extracellular- causes gradient due to law of physical chemistry. This gradient requires energy . (A- are fixed -ve charges on macromolecules e.g. proteins)
A cell - biological structure that accumulates proteins, they usually have a negative and cannot cross the membrane.
Cell membranes with no input of E are generally semi-permeable to common ions: permeable to K+, and impermeable to Na+.
How is an ion gradient formed? The permeable cation (K+) accumulates where the impermeable anion (A-) is located → high intracellular [K+]. The impermeable ion (Na+) leaks into cell slowly and is pumped out against conc gradient by Na-pump. Na+ Pump requires E in form of ATP hydrolysis → Primary active transport
The Potassium ion (K+)
Intracellular [K+] is > extracellular [K+], ∴ K+ diffuses out of the cell.
Loss of K+ → small residue -ve charge on inner side of membrane- causes ‘electrochemical equilibrium’. A membrane potential has developed.
If extracellular [K+] increases, then less diffusion between them- less steep conc gradient, this prevents an AP forming as the required membrane potential to balance the movement is less negative.
The relationship between membrane potential, Em, and extracellular [K+] calc using Nernst equation. A rise in extracellular [K+] makes membrane potential less -ve than resting membrane potential→ membrane depolarised.
Resting membrane potential
Reflects a diff in charge on either side of cell membrane- the cytoplasm side is negative in relation to the extracellular fluid. Ranges between -20 and -95 mV depending on cell type.
Ion channels
Ions move across cell membrane via ion channels (protein pores that span the phospholipid bilayer). Voltage-gated channels are activated by a small change of membrane potential e.g. an electrical stimulus. Ligand-gated channels require a neurotransmitter to open channel/ extracellular chemical binds to receptor (ion channel) on membrane.
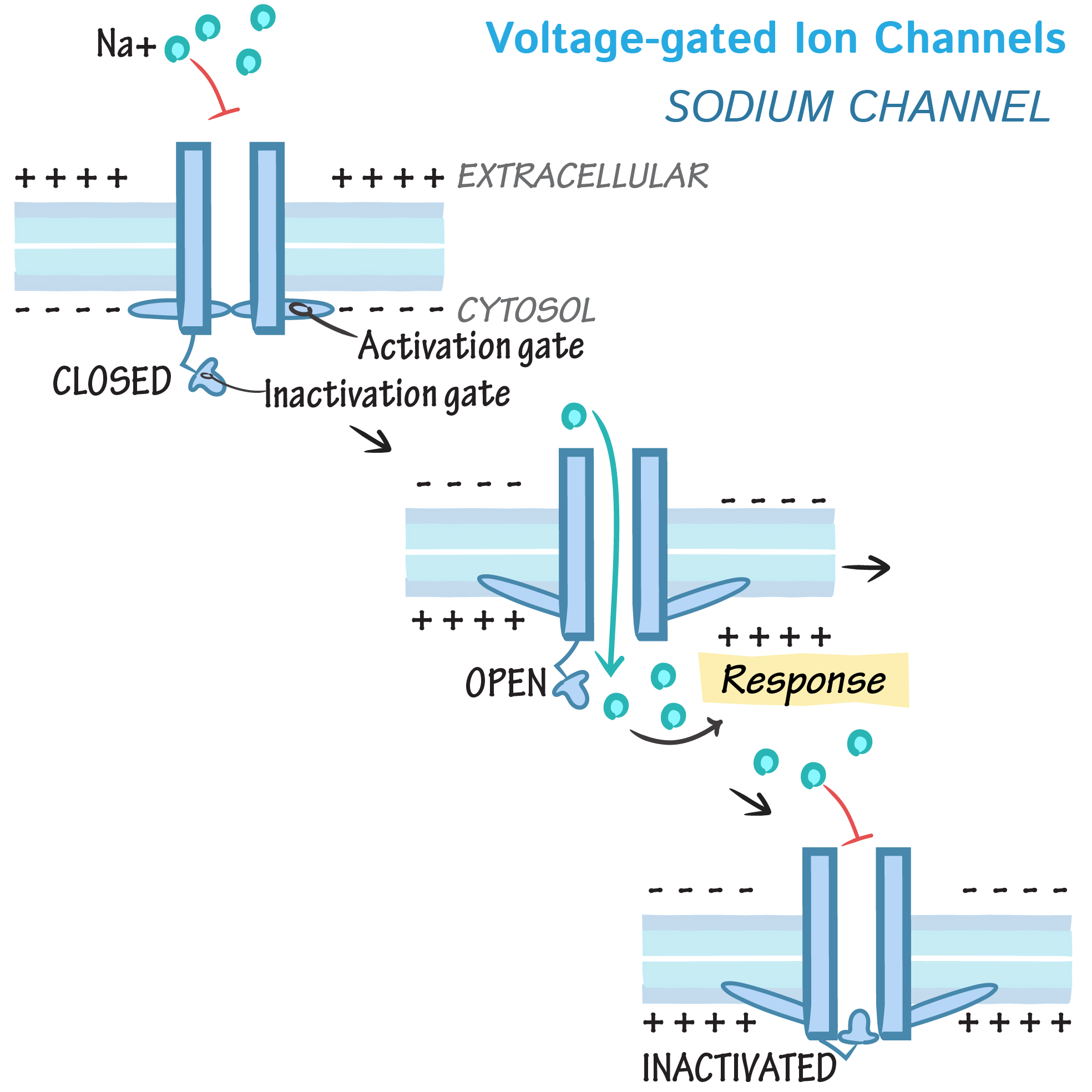
Voltage-gated ion channels
- Support APs, generated in nerve and muscle.
At resting membrane potential, activation gate closes the channel
Depolarising stimulus arrives at channel. Activation gate opens, causing Na+ to enter cell
Inactivation gate closes and Na+ entry stops
During repolarisation, caused by K+ leaving the cell, the 2 gates reset to their original position.
\n
Ligand-gated ion channels
causes shape ∆ when a ligand binds
found in muscle, nerve and some secretory cells.
They can be:
Cation-selective (mainly Na+ flow into cell), when activated cause depolarisation and excites cell.
Anion-selective (mainly Cl- flow into cell), when activated cause hyperpolarisation and makes cell less excitable.
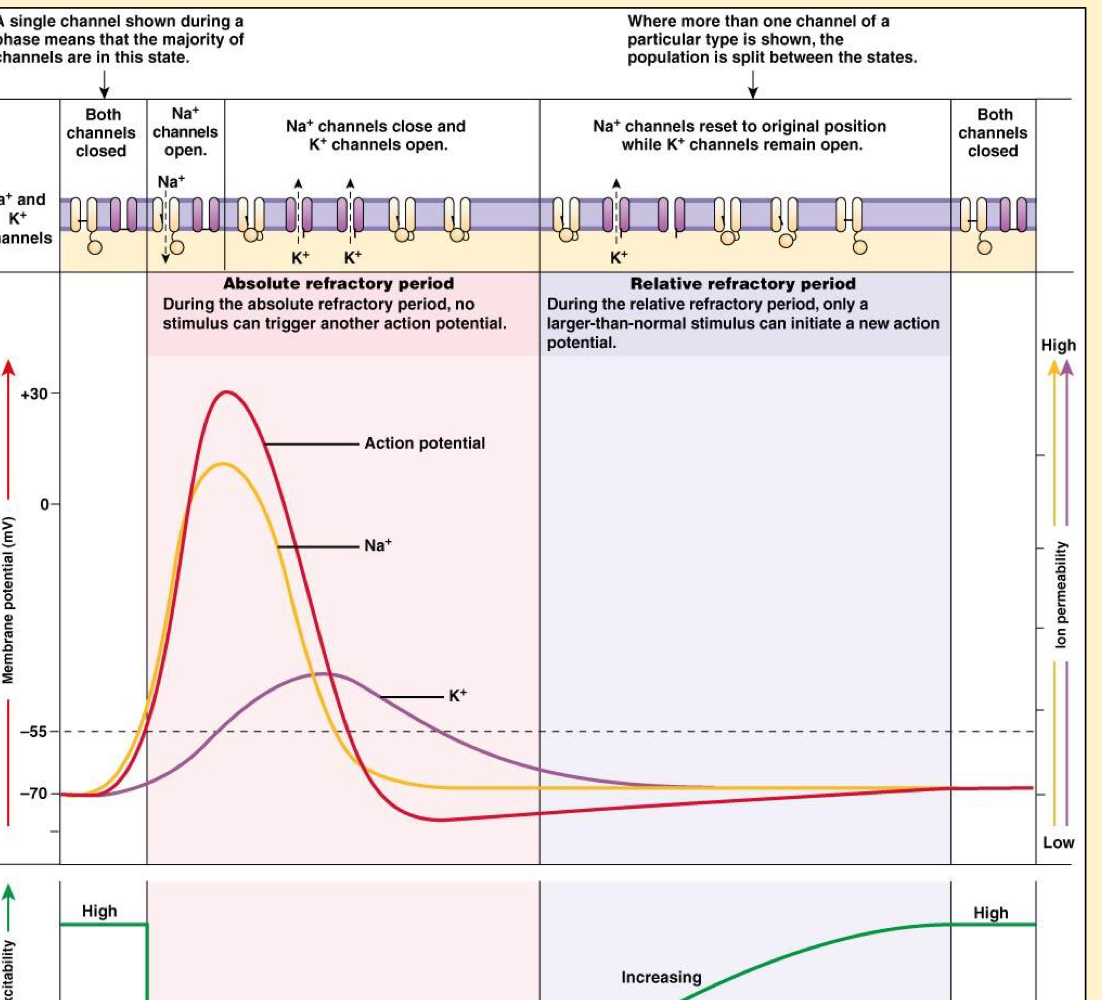
Action Potentials
- Transient depolarisation of cell
- Only excitable cells generate APs, and they have a fixed magnitude and duration.
- They transfer information, which is coded by the frequency of APs passing along a nerve e.g. from a sensory cell to the CNS
- Initiate cellular events e.g. initiate muscular contraction
- All-or-nothing law: Once an AP has been initiated, varying the stimulus strength doesn’t alter the configuration of AP
- Threshold: to initiate an AP, the cell membrane must be depolarised to a critical potential
- Alan Hodgkin and Andrew Huxley showed that the APs depolarisation was due to a increase of membrane Na+ permeability.
- To initiate an AP, a stimulus (Energy) must increase resting membrane potential, Vm to threshold potential, Vth.
- At threshold, Voltage-gated ion channels open and Na+ enters cell, generating AP.
(Small stimulus→ high excitability needed) (Large stimulus→ low excitability needed)
Resting→ threshold:
Artificial application of electrical current e.g. probe.
At synapse neurotransmitters bind to ligand-gated channels on target cell.
Spontaneously in ‘pacemaker’ cells e.g. heart.
Sensory cells: convert a stimulus to a ∆ of membrane potential of associated nerve. If stimulus large enough, threshold reached.
Trigger zone: region of cell that generates an AP- represented by reversal of membrane potential polarity.
Factors that affect AP conduction velocity (CV)
Cell diameter: CV ⬆️ as fibre diameter ⬆️.
Temperature: ⬆️ temperature generally ⬆️ CV - ∆ permeability of membrane
Myelination:
- Vertebrate nerve fibres diameter > 1μm possess a myelin sheath made of Schwann cells -surrounds the axon with breaks about every millimetre.
- Myelin greatly ⬆️ CV as the AP jumps from node to node → saltatory conduction.
As stimulus strength ⬆️, no. of APs ⬆️. AP frequency codes stimulus intensity.
Secondary active transporter - using E from Na+ gradient to fuel movement e.g. GLUT protein
Which one of the following statements about an action potential is correct?
- It is a result of Na+ and K+ entering the cell
- %%It is a transient depolarisation of a cell%%
- It can only be generated in nerves
- It is conducted more slowly in myelinated, compared to un-myelinated, nerves
- It is generated when the membrane potential becomes more negative compared to the resting value