Atomic structure and the periodic table
Atoms are the smallest part of a chemical element, comprised of neutrons, protons and electrons
Elements are substances made up of only one type of atom
Compounds are made up of two or more chemically combined elements
Mixtures consist of two or more elements not chemically combined. Chemical properties of each element involved are unchanged. can be separated by physical means
Chemical reactions always involve the formation of one or more new substances, and often involve a detectable energy change
Physical Separating techniques
Solvent = the liquid in which a solute dissolves
Solute = the dissolved substance in a solution
Solutions are formed of a solute and a solvent. Not all solutions are aqueous, because water isn’t always the solvent.
Filtration used to separate an insoluble solid from a liquid.
Filter paper has tiny pores that allow small molecules and dissolved ions through, but not larger particles of undissolved solids.
Evaporation/crystallisation separates soluble solids from liquids
When the solution is warmed, some solvent evaporates and leaves behind solute crystals.
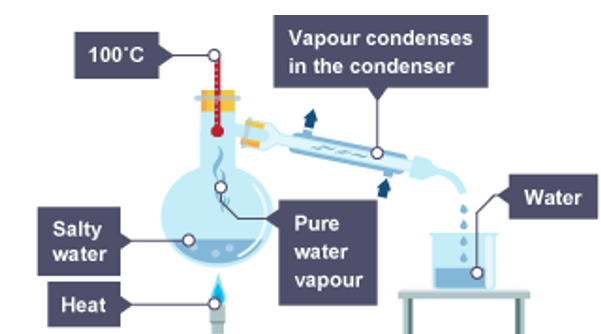
Simple Distillation separates solvents from a solution
A solute has a much higher boiling point that the solvent it’s dissolved in. so when you heat the solution, the solvent leaves the solution as a vapour (gas), cools and condenses. Remaining solution is now more concentrated
Fractional distillation separates different liquids from a mixture of liquids
Works on the same idea as simple distillation – that liquids have different boiling points, so when the mixture is heated certain liquids will vaporise at different temperatures. They move up the fractionating column (hot at bottom, cold at top), condensing when they reach a point lower than their boiling point. if the temperature at the top of the column is above the boiling point of a substance, its vapour can pass into the condenser and be collected. as the mixture is heated, eventually all the substances (fractions) will be collected
Paper chromatography separates mixtures of different coloured compounds. For more detail, see the chemical analysis topic.
The atom
Central nucleus contains protons and neutrons, surrounded by electrons in energy levels/shells
particle | Relative mass | Relative charge |
Proton | 1 | +1 |
neutron | 1 | 0 |
electron | 1/2000 (negligible) | -1 |
An element has the same amount of protons as electrons. An element is determined by the number of protons it has. Therefore,
atomic number = number of protons (and inherently number of electrons)
mass number = number of protons + number of neutrons
isotopes are forms of an element that have the same number of protons but different numbers of neutrons. Different mass number but same element
the relative atomic mass (Ar) of an element is the average mass of all different isotopes of an element.
(mass x abundance) + (mass x abundance) etc.. / total abundance
e.g:
Electron structure and configuration
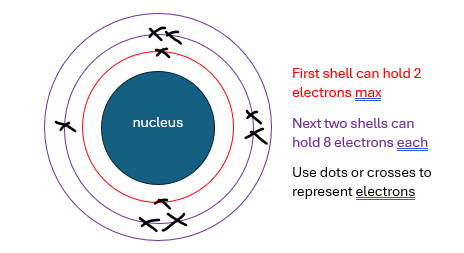
electron configuration can be represented by 3 numbers. for the above atom, it’d be 2,7 (shell 1 electron count, shell 2 electron count)
History of the atom
400 BC: Greek philosopher Democritus first coined the idea of an atom, the smallest unit of matter possible.
1800s: John Dalton adopts Democritus’ theory, establishes the first modern particle model. atom is indestructible and unchangeable.
1897: JJ Thompson’s plum pudding model: negatively charged electrons are dispersed in the surface of a massless positively charged sphere
1911: Ernest Rutherford sets up his gold foil experiment, proving the existence of a nucleus when certain particles were deflected. He declares that the nucleus is a dense mass of + charged particles and that electrons orbit the nucleus at different distances
1914: Niels Bohr develops electron shells, dictates that electrons orbit at specific distances with a set energy.
1919: the proton is discovered
1932: James Chadwick discovers the neutron, reasoning that the mass of the nucleus is too large to be solely protons.
key bits to know well
History of the periodic table
1829: Johann Dobereiner first describes the idea of triads: sorting elements into groups of 3 with similar properties, RAM and density. the elements not in triads were the transition metals.
1864: John Newlands sorted the 56 known elements in order of their atomic weight into 11 groups, using the seven intervals of music as an analogy. he was largely dismissed for grouping metals and non-metals
1869: Demitri Mendeleev arranges elements by their atomic weight and found they corresponded to properties. He left gaps where the next heaviest element didn’t fit the properties of a group, and these gaps were filled over time to get, more or less, the periodic table we use today.
atomic WEIGHT - atomic number was not discovered until 1913. there were some changes made to Mendeleev’s periodic table following this, as isotopes explained why ordering by atomic mass wasn’t always right.
Group 1 - alkali metals
single electron in outer shell
conduct electricity
low melting and boiling points that decrease down the group
soft
shiny when freshly cut
low density
more reactive down the group because the number of shells increases, so the electrostatic attraction between the nucleus and outermost electron weakens, and it’s able to be lost more easily to form a complete outer shell i.e. it’s more reactive
highly reactive, especially with water.
reaction with oxygen | reaction with chlorine | reaction with water | |
|---|---|---|---|
lithium | burns with a red flame and produces a white solid | white powder produced | fizzes on the surface then melts away |
sodium | orange flame and white solid | burns with a yellow flame and produces clouds of white powder | fizzes fast, melts into a ball, slight yellow flame |
potassium | lilac flame and white solid | burns rapidly with a lilac flame | ignites with a lilac flame |
Group 0 - noble gases
stable, full outer shells - inert and don’t react easily because of this
low boiling points increase down the group because atoms become larger - intermolecular forces thus become stronger and more energy is needed to overcome bonds
density increases down the group
Transition metals
conduct electricity
shiny when freshly cut
have ions with different charges as they can be stable in many forms
used as catalysts
form coloured compounds where most other metals will form white compounds
compared to group 1 metals:
higher melting points
higher densities
stronger and harder
much less reactive with oxygen, water and chlorine. most don’t react with oxygen or water at all. some react with the halogens, but not violently.
Group 7 - halogens
exist as diatomic molecules - two atoms joined by a covalent bond make up one molecule
melting and boiling points increase down the group because atoms become larger, intermolecular forces get stronger so more energy needed to overcome that force
become less reactive down the group as atoms get larger, electrostatic force of attraction between nucleus and outer shell decreases, it gets harder to gain the last electron to complete the outer shell
Element | Colour | State |
Chlorine Cl2 | green | Gas |
Bromine Br2 | Brown | Liquid |
Iodine I2 | Purple-black | Solid |
reactions of halogens
react with metals to form salts, held together with ionic bonds. e.g: sodium + chlorine → sodium chloride, 2Na(s) + Cl2(g) → 2NaCl(s)
react with non-metals to form halides. e.g. with hydrogen to form a hydrogen halide. these are gases at room temperature that dissolve in water to produce acidic solutions. hydrogen chloride, for example, dissolves in water to form HCl, hydrochloric acid.
fluoride, chloride, bromide, iodide
halogens also take part in displacement reactions. this means that a more reactive halogen can displace (take the place of) a less reactive halogen from a solution of its salts. as said earlier reactivity decreases down the group. so a solution of chlorine will displace iodine from a solution of it and a metal. e.g:
chlorine + potassium iodide → potassium chloride + iodine
Cl2(aq) + 2KI(aq) → 2KCl(aq) + I2(aq)
chlorine is more reactive than iodine
potassium iodide is the salt
chlorine solution displaces potassium iodide solution