Nervous Tissue – Complete Study Notes
Overview of the Nervous System
Neurons have 3 fundamental physiological properties that enable them to communicate with other cells:
1. Excitability: all cells are excitable- they respond to stimuli. Neurons exhibit this property to the highest degree
2. Conductivity: neurons responds to stimuli by producing electrical signals that are quickly conducted to other cells at distant locations
3. when the signal reaches the end of an axon, the neuron secretes a neurotransmitter that crosses the gap and stimulates the next cell
Two body-wide control systems coordinate and integrate physiology
Endocrine system: blood-borne hormones
Nervous system: rapid electrical & chemical signaling via neurons
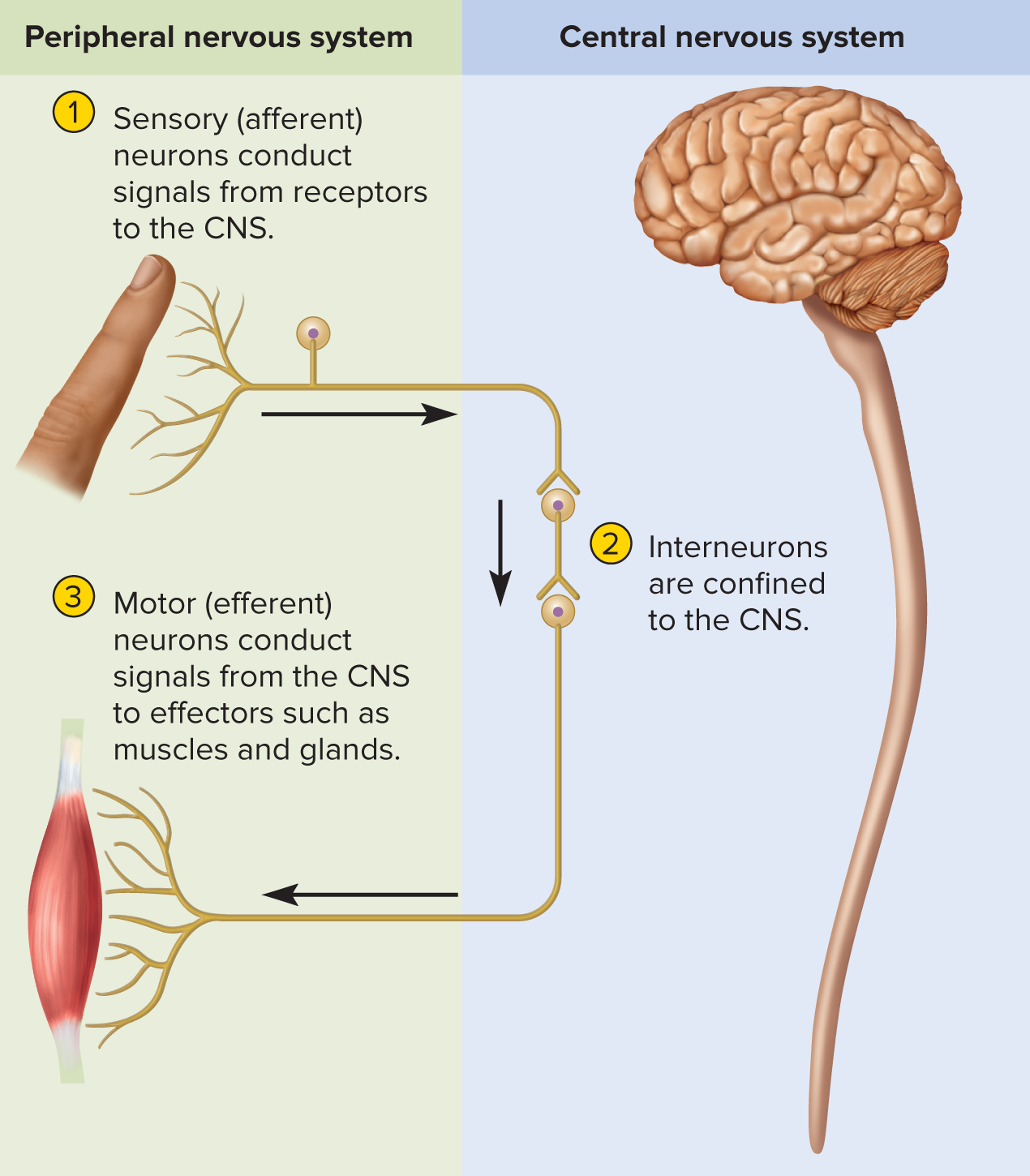
General three-step information flow
1️⃣ Sensory input: receptors → CNS
2️⃣ Integration: CNS analyzes & decides response
3️⃣ Motor output: CNS → effectors (muscles/glands)
Anatomical divisions
Central Nervous System (CNS): brain + spinal cord performs most of decision making functions
Peripheral Nervous System (PNS): nerves + ganglia
Nerve = bundle of axons in CT sheath
Ganglion = knot-like swelling housing neuron cell bodies of PNS are concentrated somata
Functional divisions of PNS (divided into sensory and motor divisions each with somatic and visceral subdivisions)
Sensory (afferent): collects and sends signals from receptors to the central nervous system
Somatic sensory: focuses on skin, muscles, bones, joints
Visceral sensory: deals with internal organs like thoracic & abdominal viscera (heart and lungs)
Motor (efferent): carries signals from the CNS mainly to gland and muscle cells that carry out body’s response
Effector: cells and organs that respond to these signals
Somatic motor: skeletal muscle (voluntary & reflex)
Visceral motor = Autonomic Nervous System (ANS)carries signals to glands, cardiac and smooth muscle with no voluntary control over these effectors)
Sympathetic:
 alertness,
alertness,  HR,
HR,  BP (“fight-or-flight”)
BP (“fight-or-flight”)Parasympathetic: calming, digestion, energy storage (“rest-and-digest”)
Enteric plexus: intrinsic GI tract network coordinating motility/secretions
has more neurons than spinal cord, for different regions of long intestine to communicate & coordinate their motility & secretion.
/
Universal Properties of Neurons
Excitability (irritability): react to stimuli
Conductivity: propagate electrical signals along membrane
Secretion: release neurotransmitter at synapse when signal arrives
Functional Classes of Neurons
Sensory (afferent): receptor → CNS
Interneurons (association): entirely within CNS, \approx 90\% of neurons; integrative “decision makers”
Motor (efferent): CNS → effectors
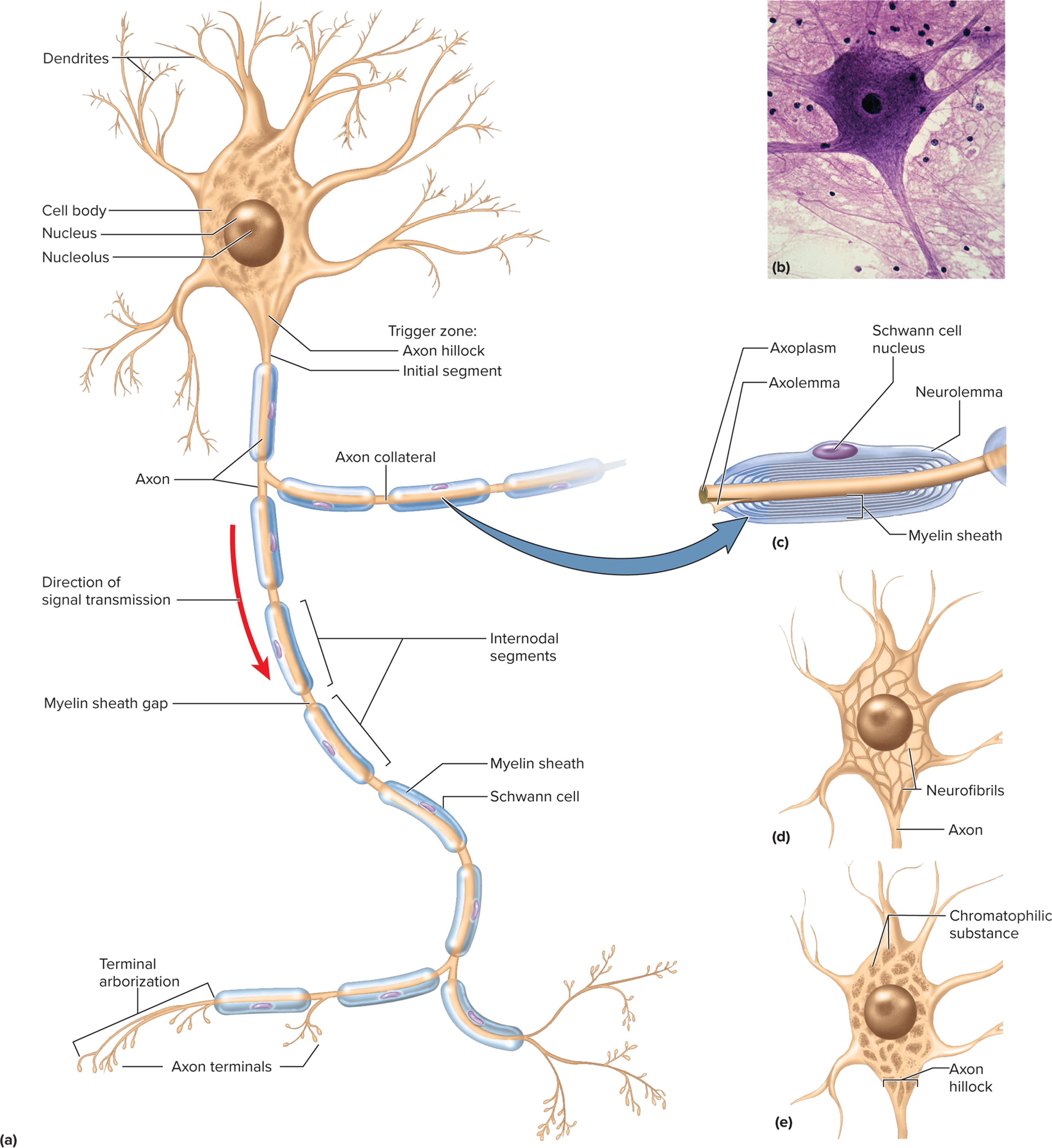
Structural Anatomy of a Neuron
Cell body (soma / neurosoma / perikaryon)
Nucleus-nucleolus being iste of rRNA synthesis and ribosome assembly, Nissl bodies (segmented RER darkly stained regions), mitochondria, Golgi, lysosomes
Cytoplasm: chromatophilic substance-free ribsosomes and rough ER
Cytoskeleton: microtubules + neurofibrils; lack centrioles ⇒ no mitosis post-adolescence
Inclusions: glycogen granules, lipid droplets, melanin, lipofuscin
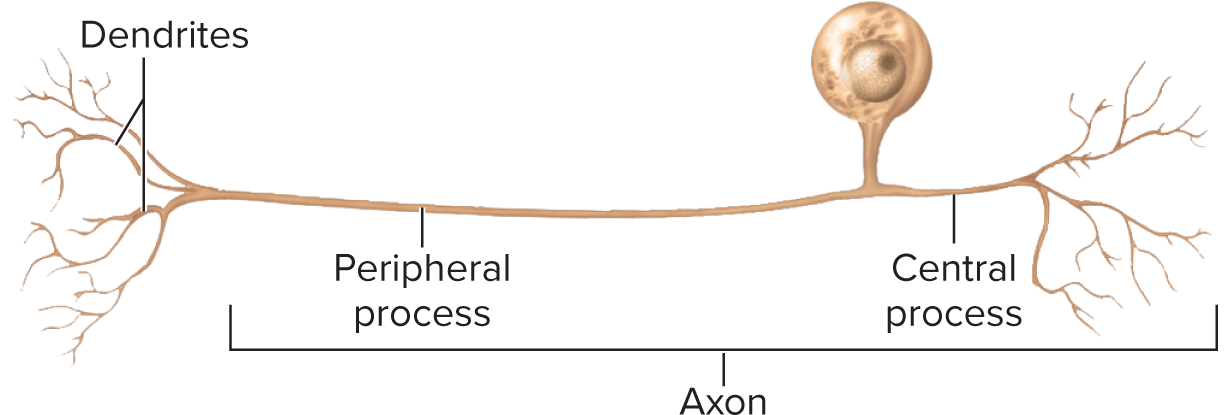
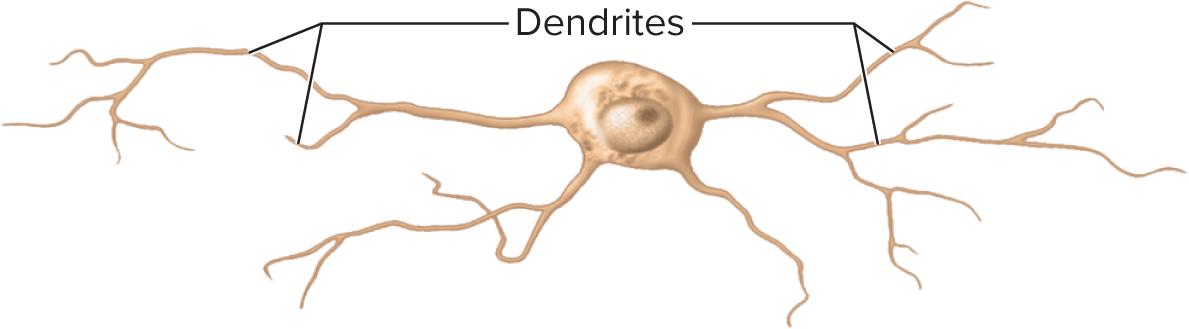
Neurites (processes reaching out to other cells are classified into two types: axons, which transmit signals away from the cell body, and dendrites, which receive signals from other neurons )
Dendrites: 1 – thousands; receive input; more dendrites = more information capacity (bare branches of a tree). Primary site for receiving signals
Body (soma): mitochondria, nucleus, rough endoplasmic reticulum
Axon (nerve fiber): single, long; originates at axon hillock carries impulses away from cell body; sends the output
Axoplasm, axolemma: cytoplasm of the axon; axolemma is the plasma membrane surrounding the axon that plays a crucial role in the conduction of nerve impulses. The axon also contains essential organelles and structures such as mitochondria and neurofilaments that support its function and integrity, ensuring efficient signal transmission throughout the nervous system.
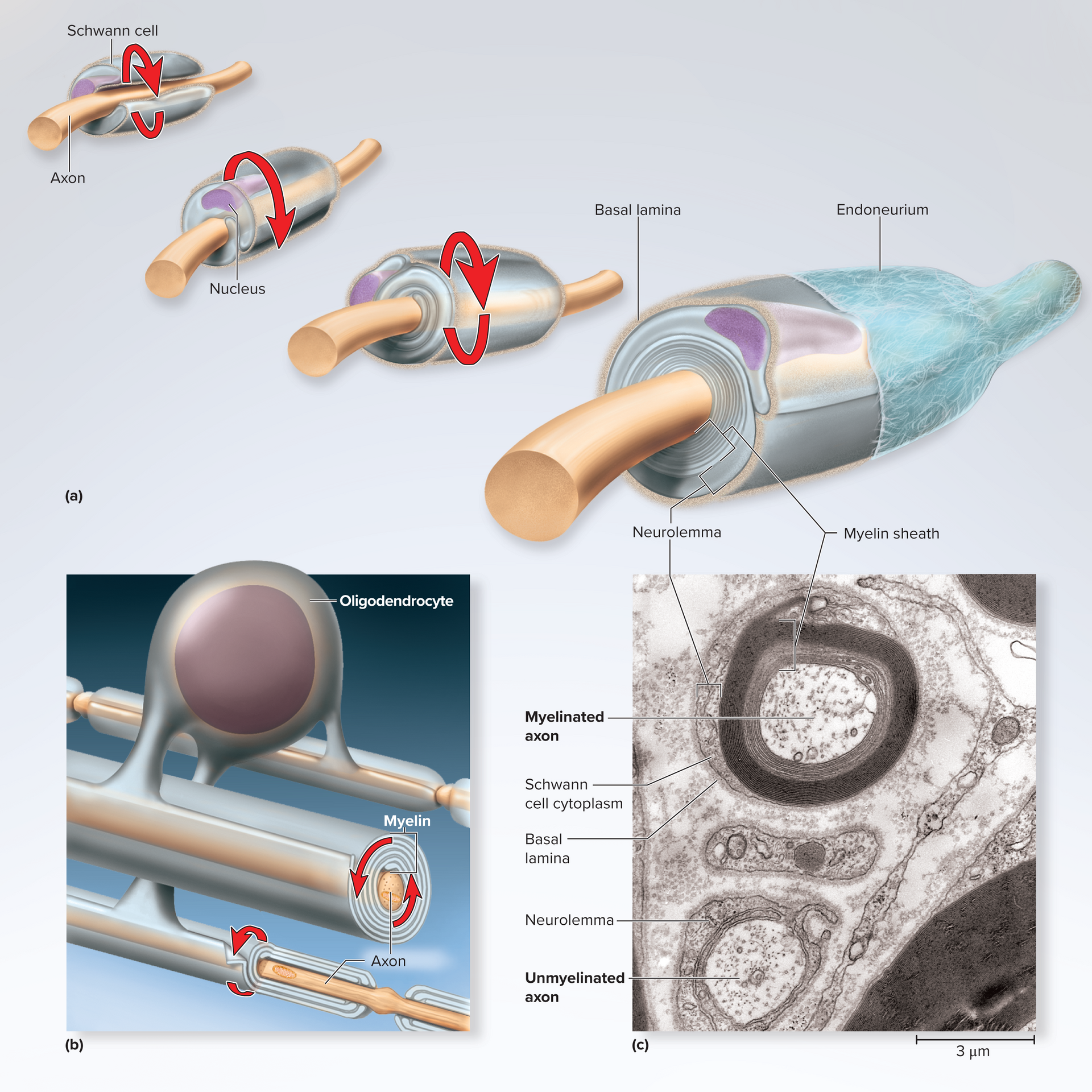
Neurolemmocytes: form myelin around PNS axon with a neruolemmocyte nucleus forming myelin sheath
Myelin sheath: insulating covering around some axons greatly increasing speed of axon signal
myelination: a process where axons are wrapped in a myelin sheath, which insulates them and significantly increases the speed of electrical signal conduction. This sheath is formed by glial cells, such as oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system, providing both protective and supportive functions.
Nodes of Ranvier: gaps in the myelin sheath that facilitate rapid signal propagation through saltatory conduction, allowing impulses to jump from one node to the next, greatly enhancing conduction speed.
Synapse: a specialized junction where axons connect with other neurons or target cells with a synaptic cleft, enabling the transmission of nerve impulses through neurotransmitter release, playing a pivotal role in communication within the nervous system.
Axon of presynaptic neuron
synaptic knob
synaptic cleft
neurotransmitter→ dendrite of postsynaptic neuron.
Neurotransmitters: chemical messengers released from synaptic vesicles at the synapse that bind to specific receptors on the post-synaptic membrane, initiating a response in the target cell and influencing neuronal communication and signal propagation.
Action Potential: a rapid change in membrane potential that occurs when a neuron sends a signal along its axon, characterized by depolarization followed by repolarization, which is essential for the transmission of signals across neurons.
Resting Potential: the electrical potential difference across the neuronal membrane when a neuron is not actively sending a signal, created by the distribution of ions (such as sodium and potassium) and maintaining the readiness of the neuron to fire an action potential.
Terminal arborization ending in synaptic boutons forming synapses
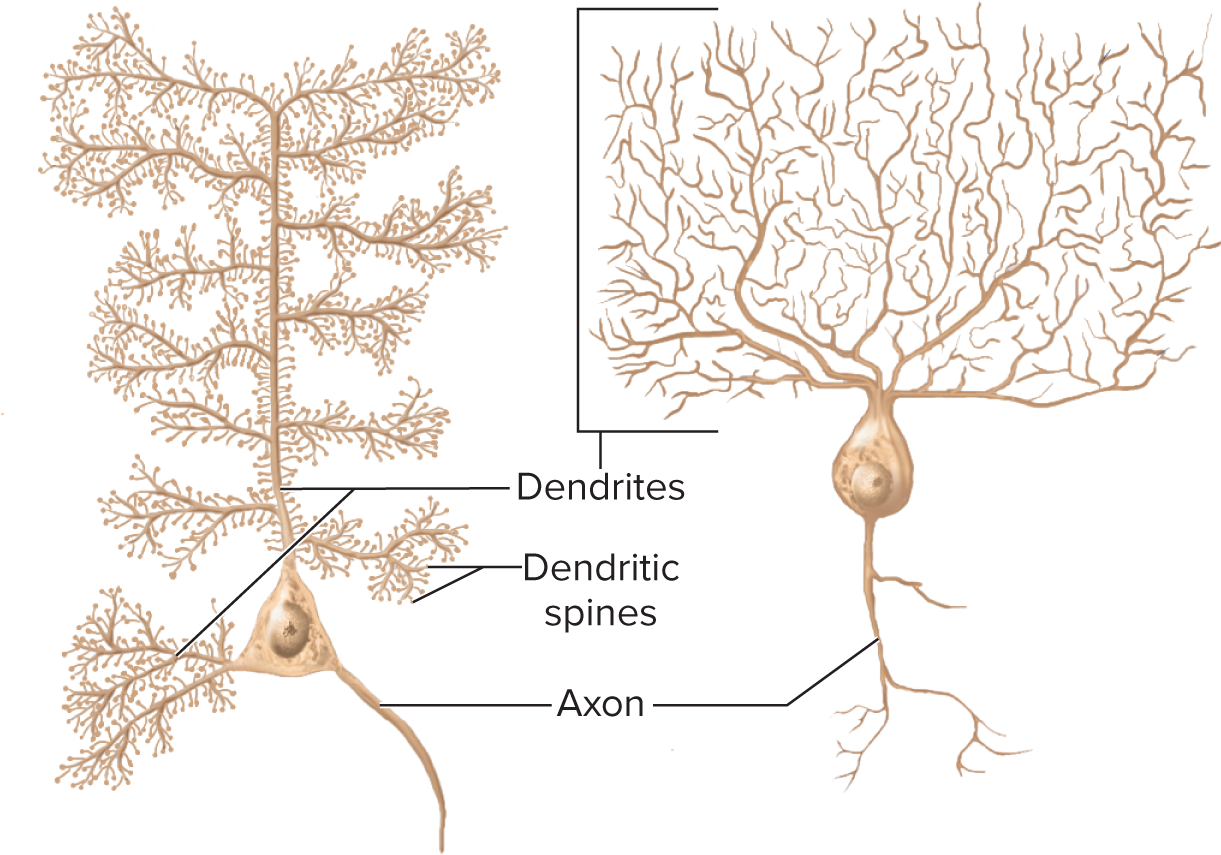
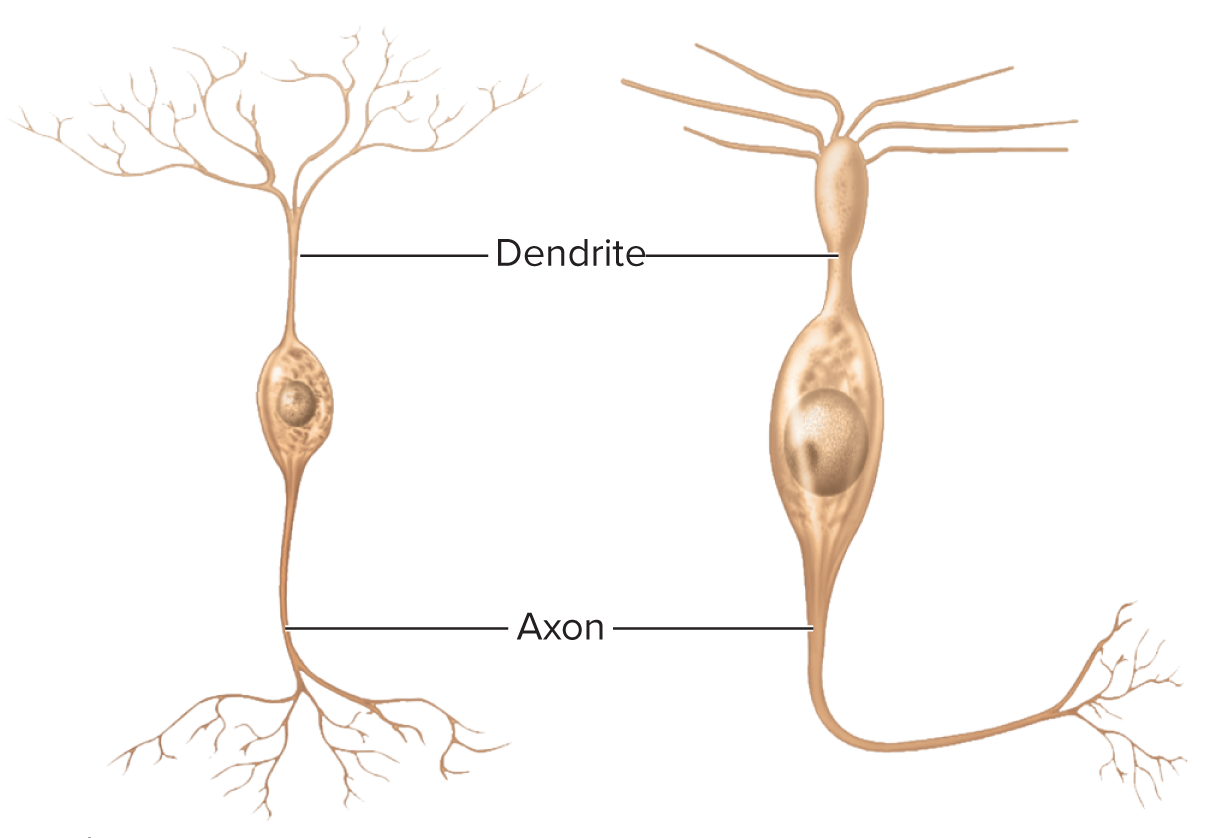
Structural neuron types
Multipolar: 1 axon + many dendrites (most CNS neurons)
Bipolar: 1 axon + 1 dendrite (retina, olfactory epithelium, inner ear)
Unipolar (pseudounipolar): single process that splits into peripheral & central axonal branches (sensory ganglia)
Anaxonic: dendrites only; no axon (retina, brain, adrenal medulla)
Multipolar neurons
Bipolar Neurons
Unipolar: involved in the senses of touch and pain
Anaxonic Neuron- Amacrine cell of retina
Axonal Transport
Bidirectional movement along microtubules via motor proteins
Anterograde (soma → axon terminal): kinesin; supplies enzymes, vesicles, organelles. Movement away from cell body, down axon, driven by kinesin
Retrograde (terminal → soma): dynein; returns used vesicles, informs soma, can carry pathogens (e.g., rabies). Movement up the axon toward the cell body driven by motor protein dynein
Speed categories
Fast: 200\text{–}400\,\text{mm/day} (both directions)
fast anterograde transport of organelles, enzymes, synaptic vesicles, small molecules
fast retrograde transport for recycled materials, some pathogens
Slow: 0.2\text{–}0.5\,\text{mm/day} anterograde only; “stop-and-go”; governs regeneration rate
Transport of enzymes, cytoskeletal components, new axoplasm
Damaged nerves regenerate at a speed governed by slow axonal
transport, which is critical for restoring functionality and repairing the nerve pathways.
Neuroglia (Supporting protecting Cells that help neurons function)
~ 86 \text{ billion} neurons in adult brain & a comparable number of glia 1:1 ratio of glial cells to neurons
Bind neurons together
Form supportive tissue framework
In fetus, guide migrating neurons to their destination
Cover mature neurons (except at synapses)
• Prevents neurons from touching each other
• Gives precision to conduction pathways
4 types of CNS glia:
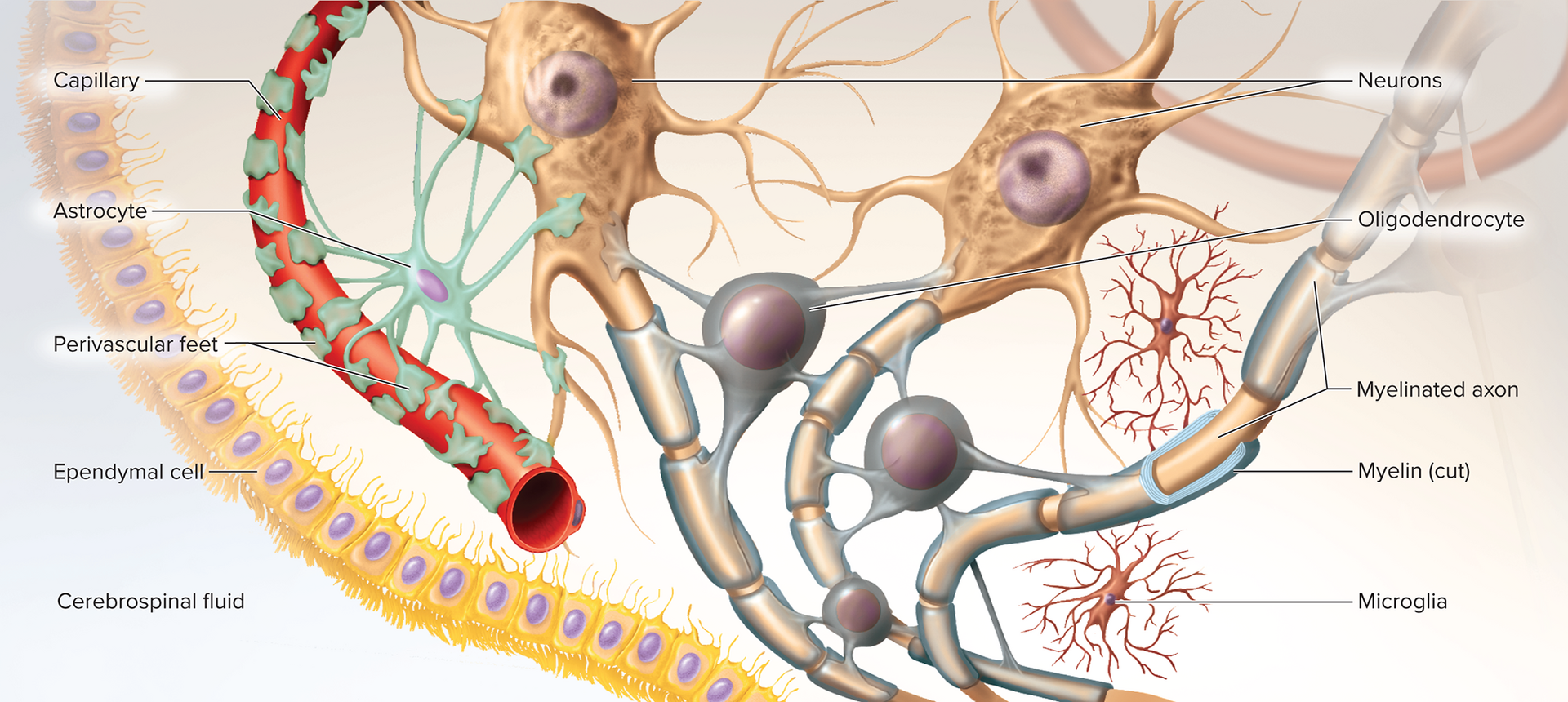
Oligodendrocytes: myelin sheaths; can myelinate multiple axons produces myelin
Ependymal cells: line ventricles, cavities of the brain and spinal cord; secrete & circulate CSF (ciliated)
Microglia: immune surveillance; phagocytize debris & microbes, dead nervous tissue
Astrocytes (most abundant)
Structural framework, perivascular feet induce \text{BBB} (blood-brain barrier)
Regulate blood flow by attaching to capillary bringing oxygen, and diffuse or filter into neuron , & neurotransmitter uptake, release lactate for energy, secrete nerve growth factors, influence synaptic signaling, regulate composition of tissue fluid
Repair scarring (astrocytosis / sclerosis)
Peripheral (PNS) glia cells, found only in peripheral nervous system
Schwann cells (neurolemmocytes): form neurilemma around all PNS fibers and myelin envelop axons of PNS, form myelin in PNS; aid regeneration of damaged fibers
Satellite cells: surround somata in ganglia; electrical insulation & chemical regulation
Clinical note: Gliomas—malignant tumors from glia grow rapidly; BBB blocks chemo ⇒ treated with surgery/radiation
Brain tumors arise from:
meninges (protective membranes of CNS)
Metastasis from nonneuronal tumors in other organs
Glial cells that are mitotically active throughout life
Myelin Sheath
spiral layers of insulation around an axon
Formed by Schwann cells in PNS, oligodendrocytes in
CNS20 % protein, 80 % lipid (high insulation value)
Production of sheath is called myelination
Begins during fetal development, proceeds rapidly during
infancy, complete by late adolescence
PNS myelination (Schwann cell) production of sheath
Cell winds repeatedly around one internode; outermost layer = neurilemma (contains nucleus & cytoplasm)
External basal lamina + endoneurium CT
Myelination
CNS myelination (oligodendrocyte)
Each process wraps part of several axons; no neurilemma or endoneurium; spiral inward
During myelination, nucleus cannot migrate around the
axon like a Schwann cell doesMust push newer layers of myelin under the older ones, so
myelination spirals inward toward axonNo neurilemma
Segmentation terminology for both PNS and CNS, myelin sheath is segmented
Many Schwann cells (PNS) or oligodendrocytes (CNS) are
needed to myelinate one axonEach segment of axon wrapped by different glial cell
Myelin sheath gap (node of Ranvier)—gap between
segmentsInternode segments: myelinated segment encircled by schwann cells
Initial segment—bare section of axon between the axon
hillock and the first glial cell: Initial segment + axon hillock = trigger zoneNode of Ranvier: gap between myelin sheaths (voltage-gated channel hot-spot) where conduction/repolarization is conducted on axon making it faster since it doesn’t have to go through the internode segments
Demyelinating diseases
Multiple Sclerosis: autoimmune oligodendrocyte destruction ⇒ plaques, conduction block, adult onset
Tay–Sachs: hereditary GM2 ganglioside accumulation in myelin ⇒ neural dysfunction, fatal by age 4
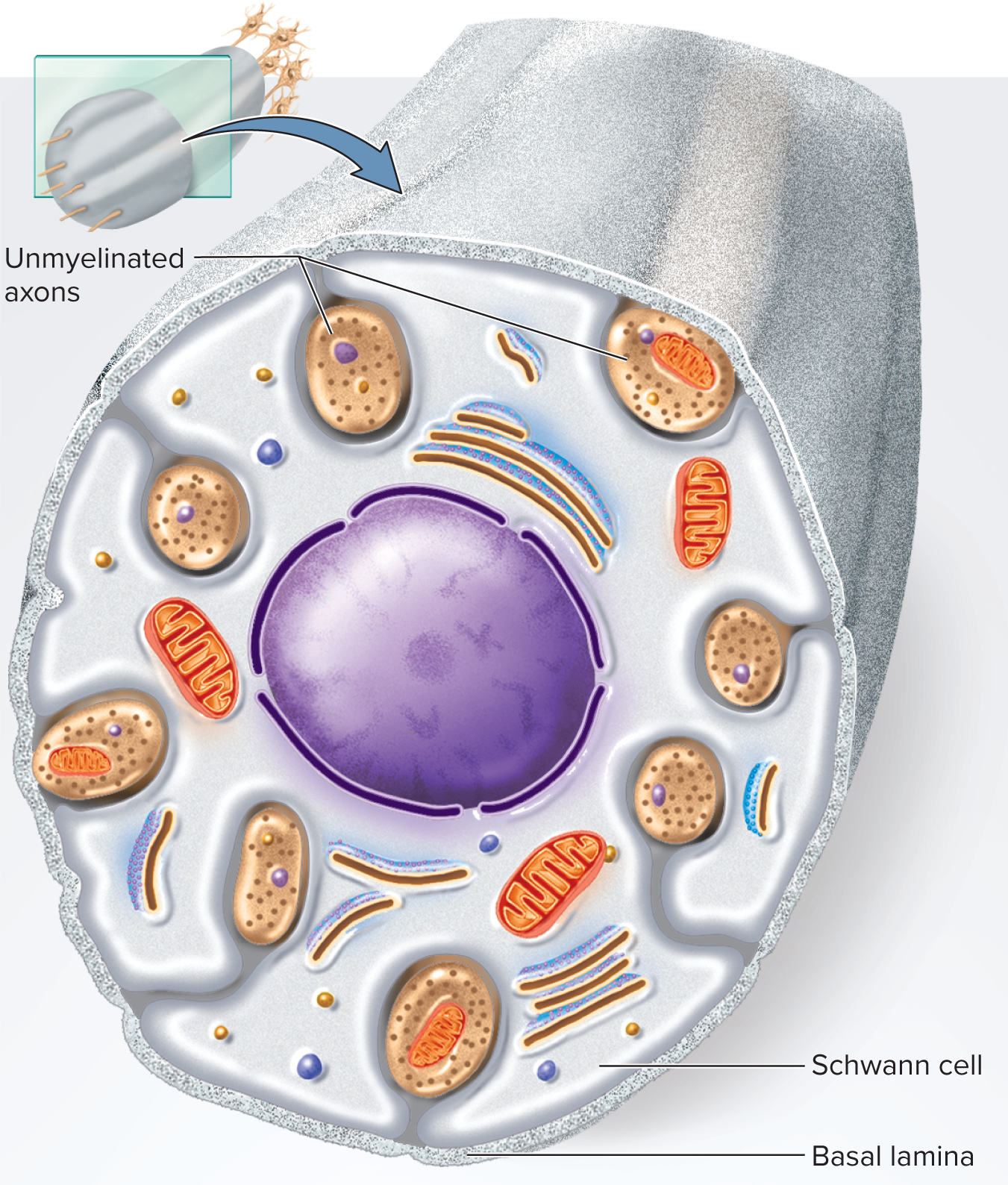
Unmyelinated Axons & Conduction Speed
PNS: one Schwann cell envelopes several axons in surface grooves; single wrap = neurilemma
Many axons in CNS and PNS are unmyelinated
• In PNS, Schwann cells hold small unmyelinated axons in
surface grooves
• Membrane folds once around each axon; does not spiral repeatedly
around it
• This wrap serves as the neurolemma
• Basal lamina surrounds Schwann cell along with its axonscontinuous Conduction velocity factors
Diameter: larger ⇒ lower resistance ⇒ faster
Myelin: saltatory conduction greatly accelerates
Examples
Small unmyelinated: 0.5\text{–}2\,\text{m/s}
Small lightly myelinated: 3\text{–}15\,\text{m/s}
Large myelinated: up to 120\,\text{m/s}
unmyelinated axons
Nerve Regeneration (PNS)
Possible if soma intact & some neurilemma present
Requires Endoneurium for nerve fiber regeneration
Steps
Distal stump degenerates; macrophages clean debris (Wallerian degeneration)
Soma swells; Nissl bodies disperse; nucleus off-center
Axon sprouts multiple growth cones
Schwann cells + basal lamina + endoneurium form regeneration tube → guide growth
Axon reestablishes synapse; soma returns to normal; muscle fibers regrow
Limitations: ≈ 1\text{–}2\,\text{mm/day}; may miswire; CNS axons lack neurilemma ⇒ poor regeneration
Damaged nerve fibers in the CNS cannot regenerate at all but suffers less trauma than the PNS being enclosed in bone
Regeneration in the PNS is facilitated by schwann cells, may be slow but not always perfect
Electrophysiology of Neurons
Neural communication is based on electrical potentials and
currentsElectrical potential—difference in concentration of charged particles between one point and another. A form of potential energy that can produce current
• Type of potential energy; measured in volts
• Under some conditions, can produce a currentCurrent—flow of charged particle from one point to another like ions
Polarized: refers to a cell membrane across which there is a separation of electrical charge, so that one side is more positive and the other side is more negative
Resting Membrane Potential (RMP)
Resting membrane potential (RMP)—charge difference across plasma membrane
Typical neuron RMP: -70\,\text{mV} in an unstimulated, resting neuron (inside negative)
Negative value indicates more negatively charged particles on
inside of membrane compared to outside
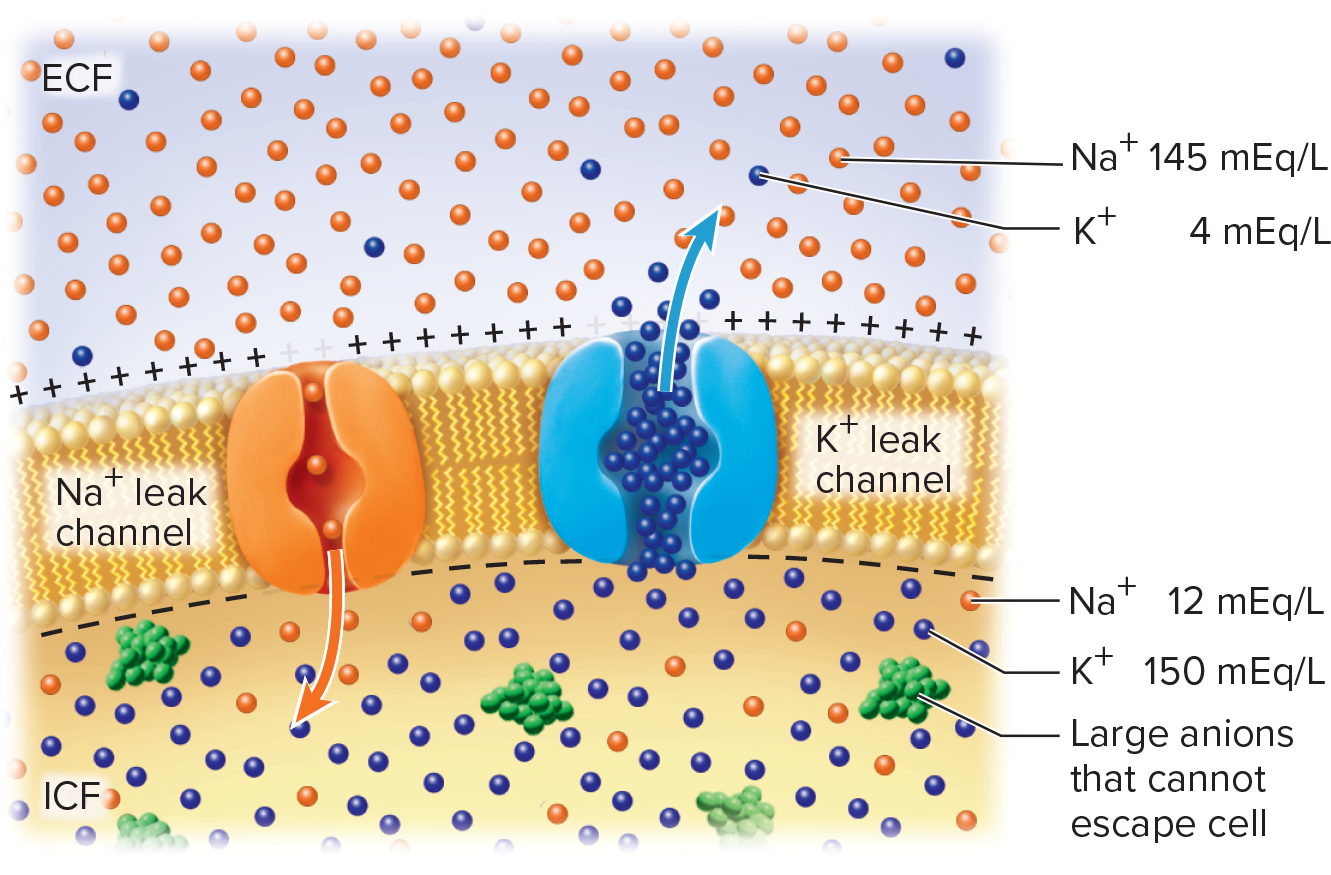
Electrical currents in the body created by flow of ions+ + (Na , K , etc.)
through gated channels in the membraneions are unequally distributed between extracellular fluid
(ECF) and intracellular fluid (ICF)Ionic contributors
[K^+]_{\text{ICF}} high; membrane highly permeable via leak channels ⇒ K+ efflux until equilibrium
[Na^+]_{\text{ECF}} high; limited permeability; small Na+ influx slightly offsets negativity
\text{Na}^+/\text{K}^+ ATPase: 3 Na+ out / 2 K+ in; maintains gradients & ≈ -3\,\text{mV} of RMP; consumes 70\% of neuronal ATP
Potassium (K+ ) has greatest influence on RMP due to the plasma membranes high permeability to K+
•K+ is more concentrated in ICF compared to ECF
• Cell membrane more permeable to K+ (through leak channels) than to other ions
• As K+ leaks out, inside of membrane becomes more negative, creating slight local accumulation of the K+ on the outside of the membrane relative to the inside
• Resulting electrical attraction brings K+ back in
• Equilibrium is reached: no net movement of K+ occurs when
tendency foe K+ to exit (down its concentration gradient) equals
tendency for K+ to enter (by electrical attraction)Sodium ( Na^{+} ) also influences the RMP but is much less permeable to Na+ than to K+
Na^{+} is 12x more concentrated in ECF compared to ICF, but membrane is much less permeable to Na^{+} than to K^{+}
Na^{+} diffuses into the cell, down its concentration gradient and attracted electrically, but it’s a relatively small amount
Cancels some of the negative charge, reducing the voltage across the membrane
Sodium-potassium(Na /K )+ + pump compensates for the continual leakage of Na+ and K+
• Moves Na+ out of the cell and brings K+ into the cell—
maintains their concentration gradients
• Contributes about -3 mV to the RMP
• Works continuously and requires ATP
• 70% of the energy requirement of the nervous system
ionic basis of resting membrane potential
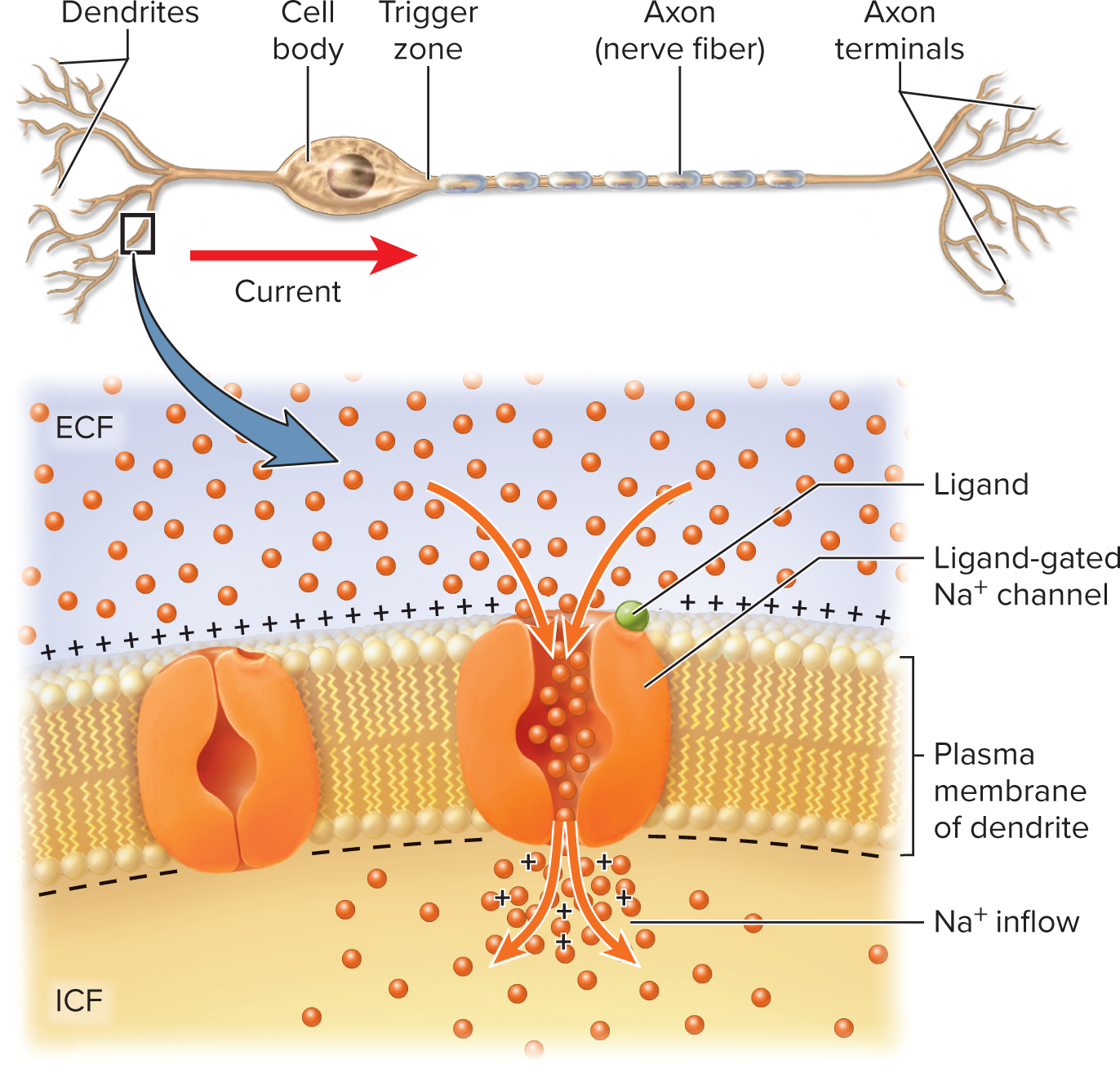
Local (Graded) Potentials
Sensory neurons can be stimulated by chemicals, light, heat, or
mechanical forces
Produced at dendrite/soma by stimuli (chemicals, light, heat, stretch)
Features
Opens Na+ channels, allows Na+ to enter
Entry of positively charged Na+ makes membrane potential less
negativeGraded (proportional), decremental (fade with distance), reversible, excitatory (depolarize) or inhibitory (hyperpolarize)
Polarity is reduced, voltage is less negative—called
depolarizationDepolarization: Na+ entry makes membrane less negative
spreads from the point of stimulation
Temporary, short-range change in voltage is a local potential
Characteristics of local potentials
Graded—vary in magnitude with stimulus strength
• Stronger stimuli open more Na+ channels, and they stay open longerDecremental—get weaker the farther they spread from
the point of stimulationReversible—if stimulation ceases, membrane voltage
quickly returns to normal resting potentialCan be either excitatory or inhibitory
Depolarization is excitatory—makes a neuron more likely to fire an action potential)
Hyperpolarization (membrane more negative) is inhibitory—makes a neuron less likely to produce an action potential
excitation of a neuron by a chemical stimulus
Action Potentials (AP) rapid up-and-down change in voltage
produced by the coordinated opening and closing of voltage-
gated ion channels
Only occurs where there is a high enough density of
voltage-gated ion channels (trigger zone of axon)Depolarization: refered to the upward change in membrane potential during an action potential
If excitatory local potential reaches trigger zone and is still strong enough, it opens enough voltage-gated Na +channels to generate an action potential
All-or-none spike generated at trigger zone when local potential reaches threshold (≈ -55\,\text{mV})
They do not decrease with distance (nondecremental), ensure consistent communication across long distances, they are unstoppable with irreversable nature
Steps of an action potential:
Local potential spreads to axon hillock
Voltage at axon hillock must reach threshold—the
minimum voltage to open voltage-gated channels (around
−55 mV)Voltage-gated Na+ channels open quickly
Voltage-gated K+ channels open more slowly
Na+ enters and depolarizes membrane further, quickly surpassing 0 mV
Na+ channels become inactivated, begin closing
Voltage peaks at +35 mV by the time Na+ inflow ceases
Membrane polarity has reversed—now more positive on the inside and negative on the outside
The voltage-gated K+ are fully open, K+ flows out of cell and
membrane becomes more negative again— repolarizationK+ continues to exit and produces a negative overshoot
hyperpolarization) 1 to 2 mV more negative than the original RMPMembrane voltage returns to RMP as Na+ leaks into the cell
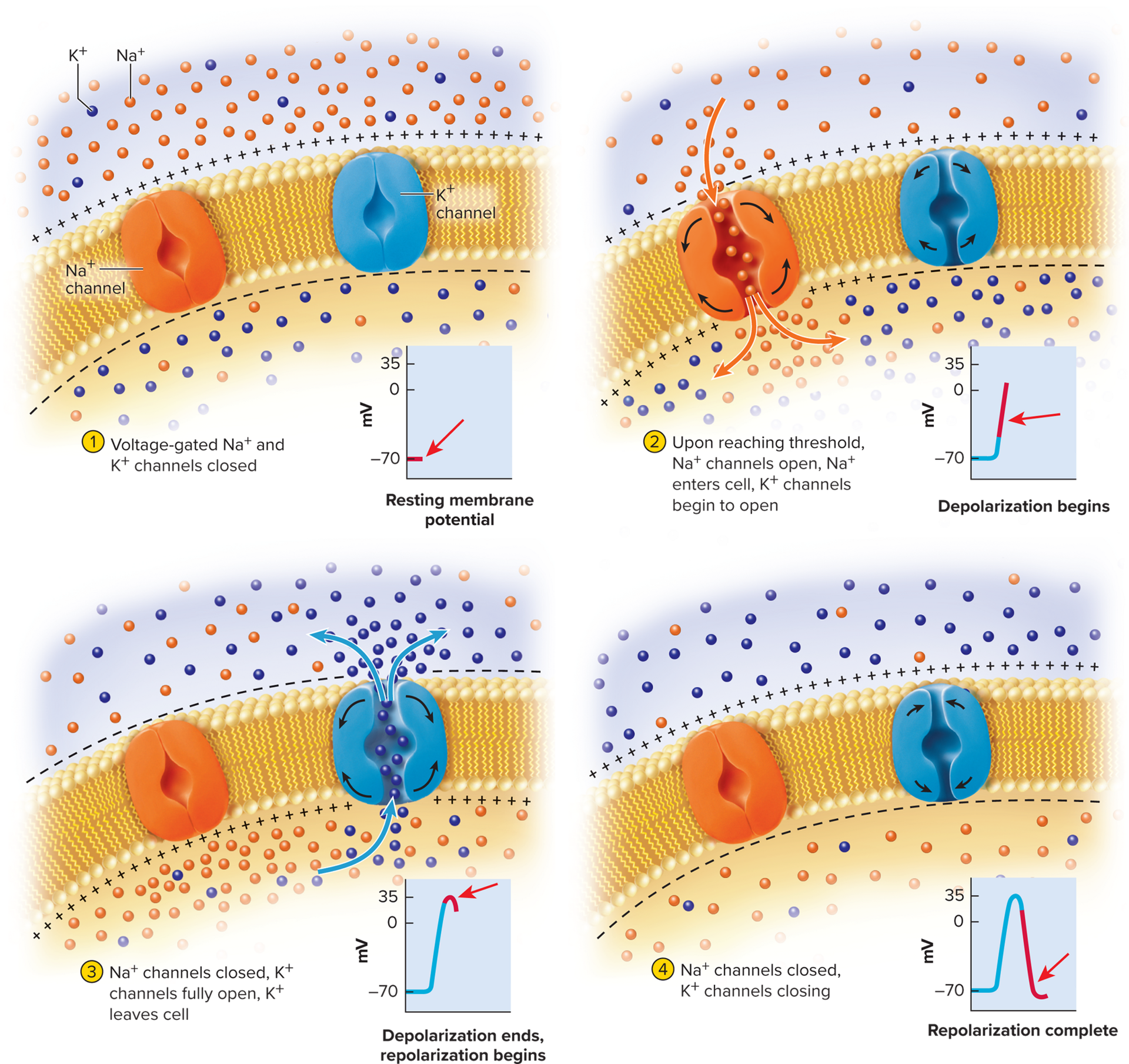
The concentrations o Na+ and K+ on either side of the membrane do not change significantly during an action potential
Movement of only a few ions can have a large effect on the
membrane potentialOnly about one in a million ions crosses the membrane during
an action potentialOnly the thin layer of ions close to the membrane is affected
Even after thousands of action potentials, the cytosol still has a
higher concentration of K+ and a lower concentration of Na+ than the ECFCharacteristics of action potentials:
All-or-none law—if threshold reached, neuron fires up to
maximum voltage; if threshold not reached, it does not fireV}Non decremental—do not get weaker with distance
Irreversible—once started, an action potential travels all
the way down the axon cannot be stopped
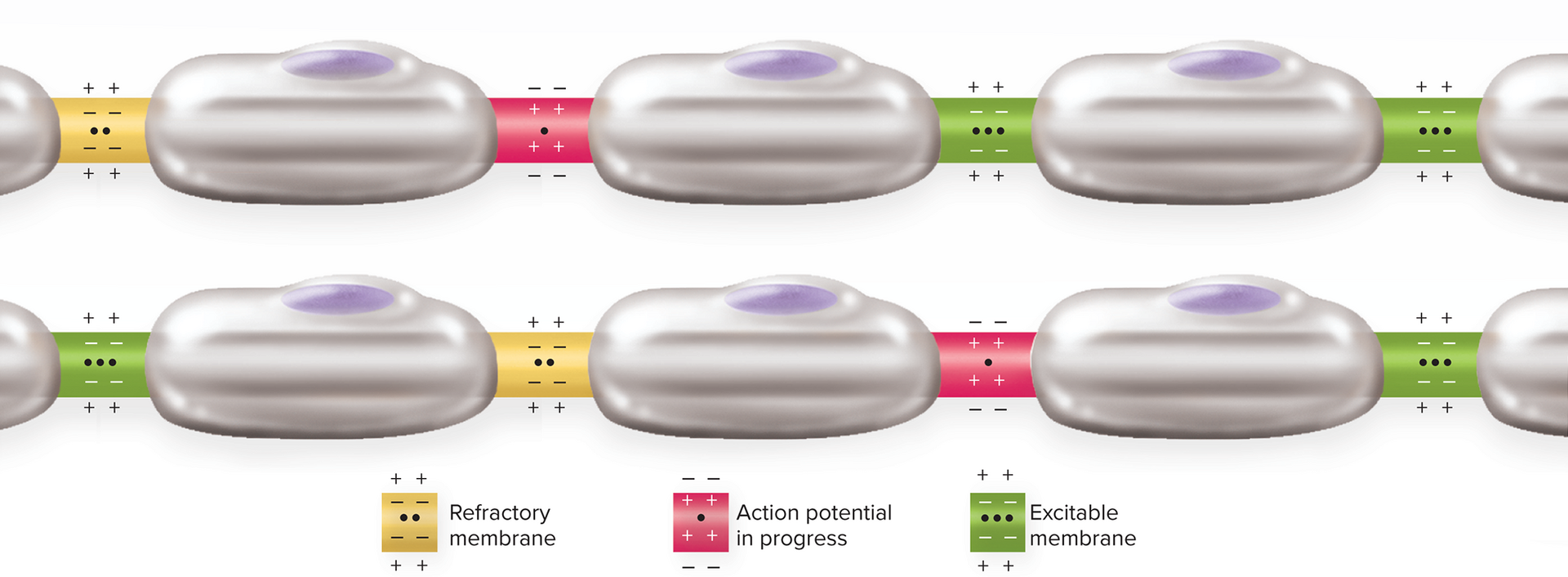
Refractory Periods
Refractory period—period of resistance to stimulation; has
two phases:Absolute refractory period: no new AP (caused by inactivation of voltage gated Na+ channels inactivated)
Relative refractory period: an unusually stronger stimulus needed to trigger a new AP may fire (during hyperpolarization, larger depolarization or local potential is required to reach threshold)
no stimulus of any strength will trigger new action potential
occurs during depolarization and repolarization phases of action potential
refers only to a small patch of the membrane; other parts of the neuron can still be stimulated while a small area of it is refractory
Signal Conduction
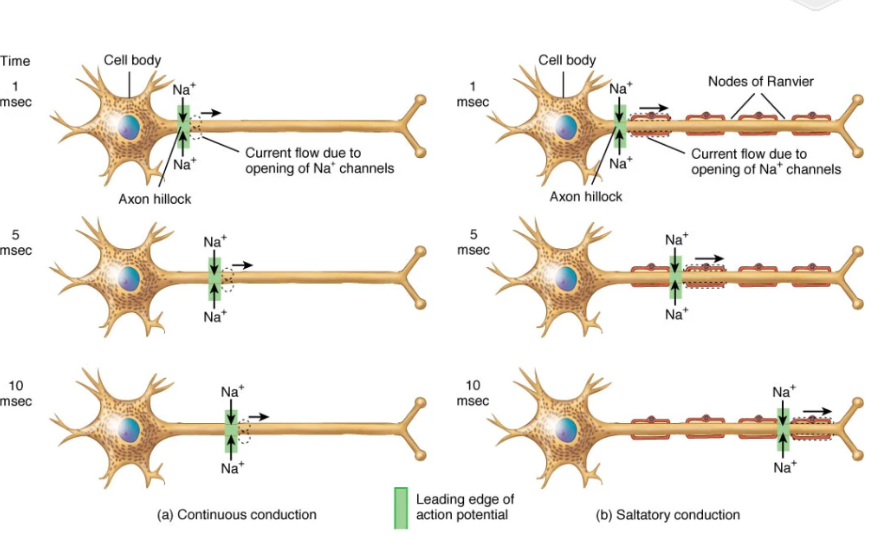
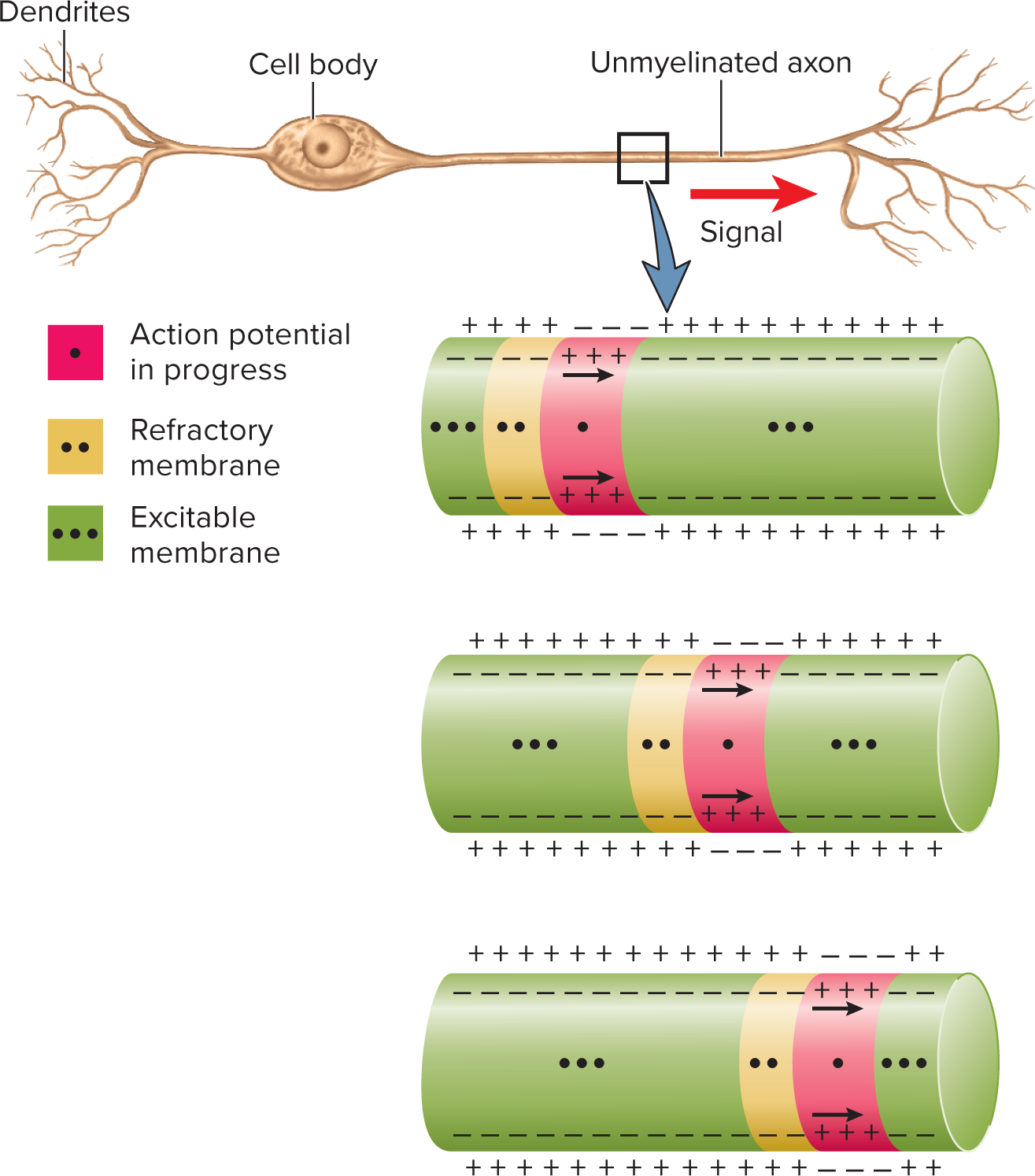
Unmyelinated axons and continuous conduction:
Unmyelinated axons have voltage-gated channels along
their entire lengthAction potential at trigger zone causes Na+ to enter axon and diffuse into adjacent regions
Depolarization opens voltage-gated channels
Opening of voltage-gated ion channels results in a new action potential which then allows Na+ diffusion to excite membrane immediately distal to that
Chain-reaction continues down axon:
Continuous (unmyelinated): sequential opening of channels along entire axolemma; relatively slow
Saltatory (myelinated): APs only at nodes; internodal Na+ diffusion renews spike at next node; fast & energy-efficient
continuous conduction of a nerve signal in an unmyelinated axon
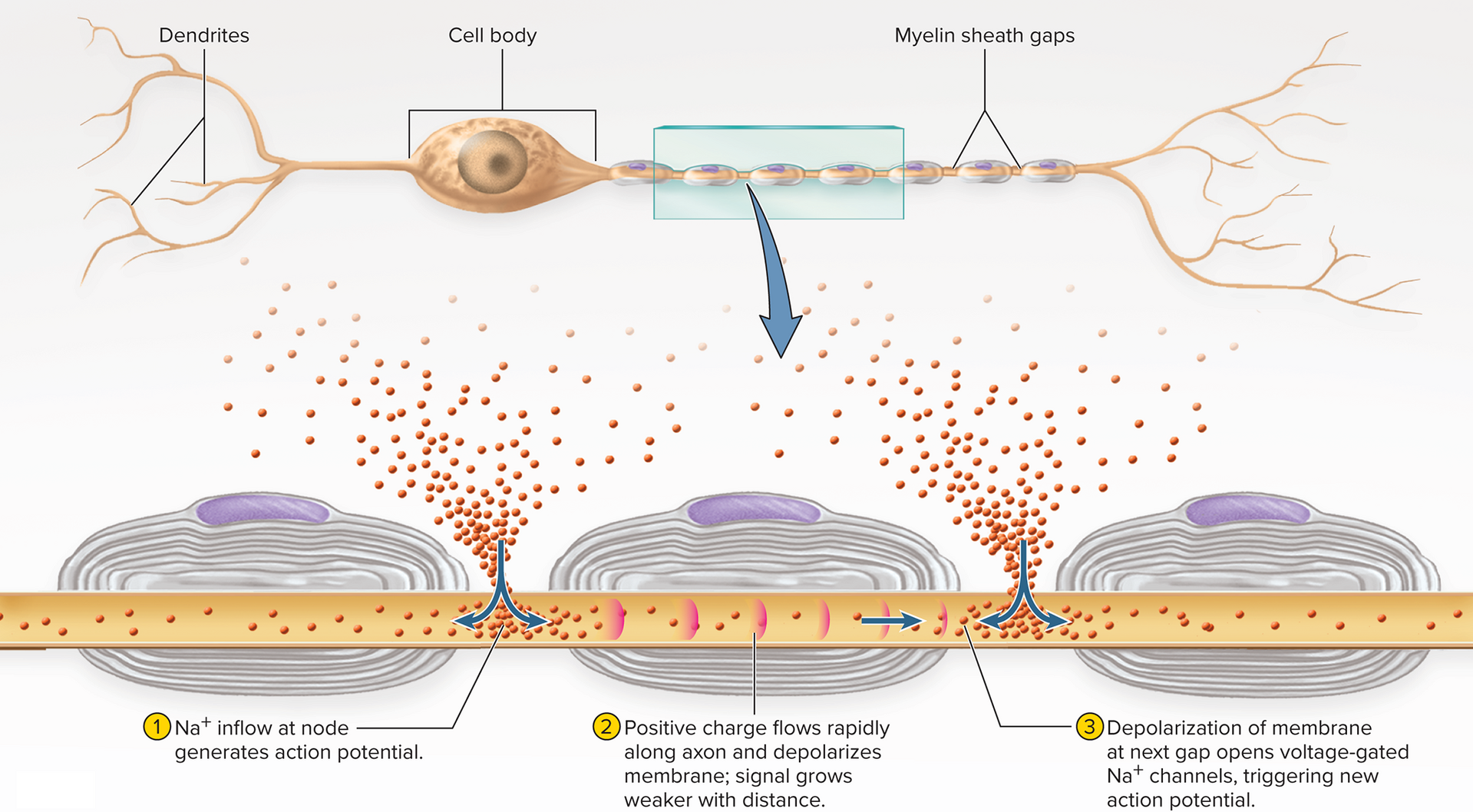
Myelinated axons and saltatory conduction:
Action potentials can only be generated at the nodes,
where voltage-gated ion channels are concentratedElectrical signal must spread passively between nodes
Signal passes very quickly, but strength decreases (similar to a local potential)
When signal reaches the next node it is still strong enough
to depolarize the membrane to thresholdVoltage gated Na+ channels open and a new, full strength action potential occurs
Action potential seems to “jump” from node to node
Moves faster through “insulated” segments covered with myelin
Slows down when it reaches the bare axon of the nodes
Synapses
point where an axon terminal meets the next cell (another neuron, gland cell, muscle cell)
For neuron-to-neuron synapses:
• Action potential arrives at end of axon of presynaptic neuron
• Presynaptic neuron releases neurotransmitter
• The postsynaptic neuron responds to itElectrical synapses: these are direct connections between neurons that allow ions and small molecules to pass through gap junctions, enabling rapid signal transmission without the need for neurotransmitter release.
For chemical synapses: neurons communicate by neurotransmitters, also site of learning and memory, target of many prescription drugs, and site of action of drugs of addiction among other things
• Neurotransmitters diffuse across the synaptic cleft •
Bind to specific receptors on the postsynaptic membrane
Trigger a response in the postsynaptic neuron, which may be excitatory or inhibitory, influencing whether the action potential will propagate.
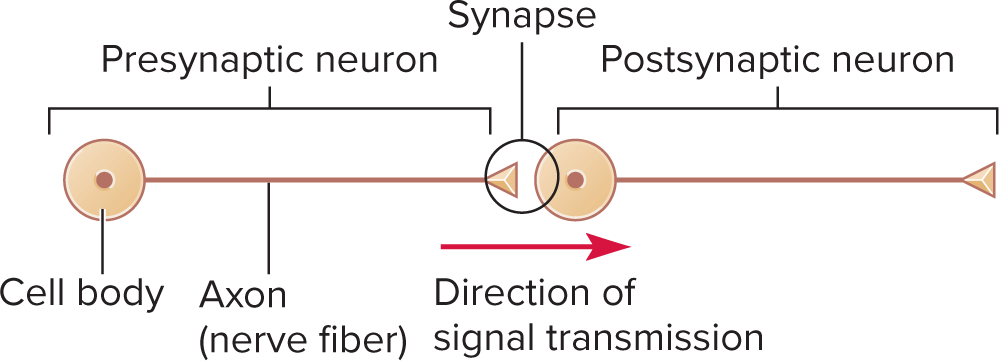
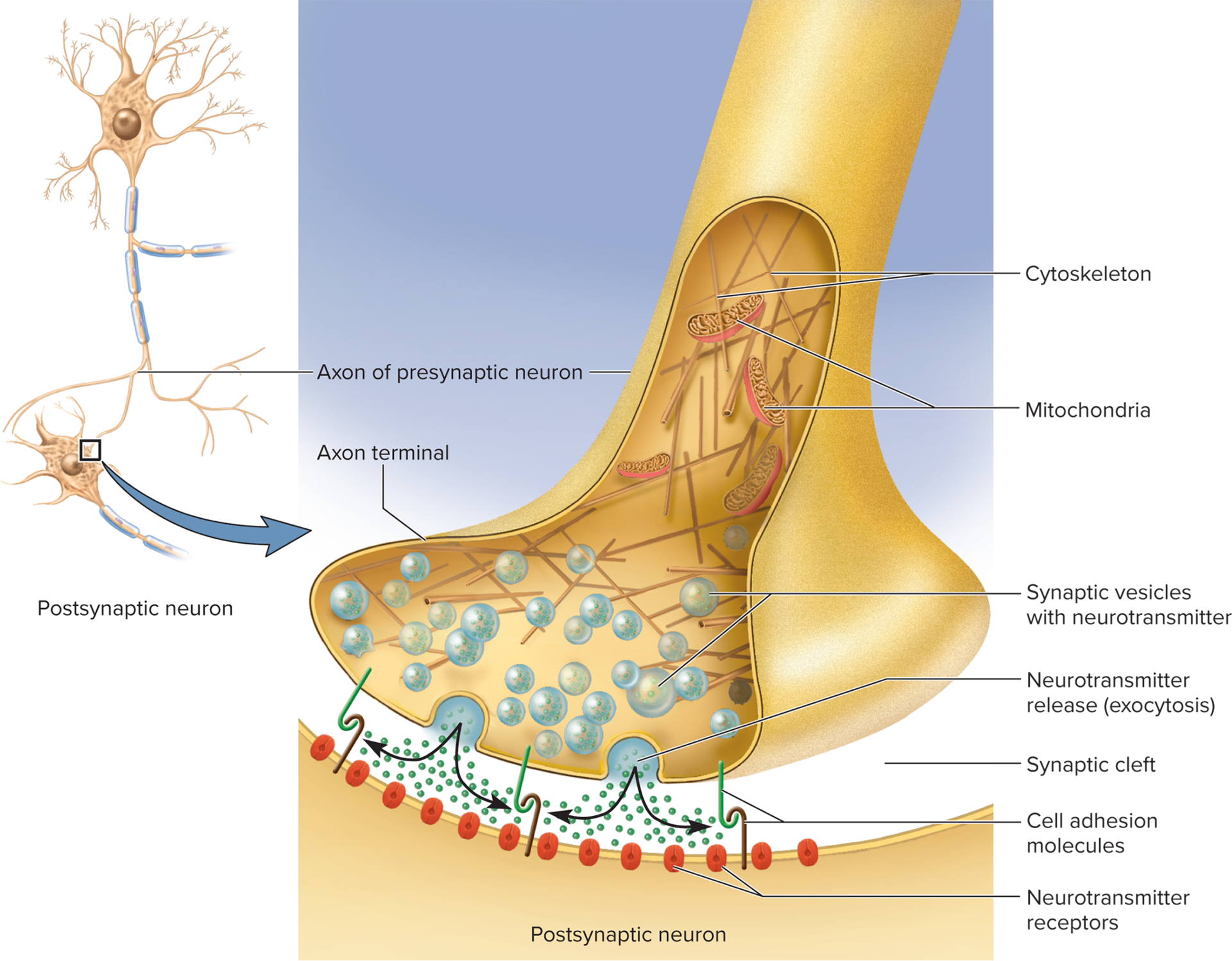
Basic Anatomy
Presynaptic axon terminal: synaptic vesicles docked at active zones
Synaptic cleft: gap between presynaptic neuron and
postsynaptic neuron≈ 20\,\text{nm} gap with CAMs linking cells
Each neuron has cell-adhesion molecules (CAMs) reaching
into the cleftCAMs link the two neurons together
Axon terminal of presynaptic neuron contains synaptic vesicles
containing neurotransmitterMany vesicles are docked on release sites on plasma
membrane ready to release neurotransmitter
Postsynaptic membrane: receptor & ion channel cluster (postsynaptic density) contains a postsynaptic density of neurotransmitter receptors and ion channels
Ligand-gated ion gates open when neurotransmitters bind to
them
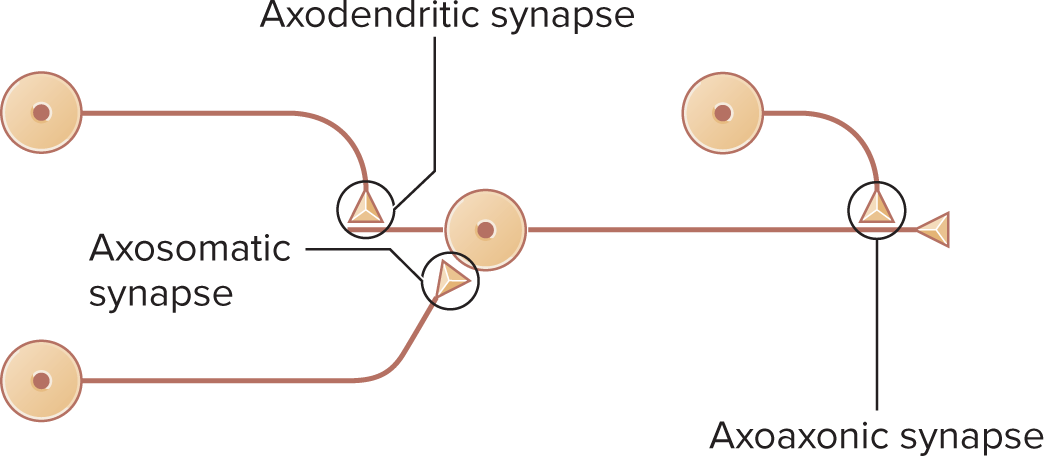
Axodendritic, axosomatic, axoaxonic variants (last enables presynaptic modulation)
axodendritic: synapse where the axon of one neuron forms a connection with the dendrite of another neuron
axosomatic: synapse where the axon connects directly to the soma (cell body) of another neuron
axoaxonic: synapse where the axon of one neuron connects to the axon of another neuron, often playing a crucial role in presynaptic inhibition or facilitation.
These synaptic connections are essential for neural communication and influence overall neuronal activity.
dendrodendritic: a synapse where the dendrites of two neurons connect with each other, allowing for local communication and integration of signals between neighboring neurons.
axoaxonic: a type of synapse where the axon of one neuron forms a connection with the axon of another neuron, allowing for the modulation of neurotransmitter release and contributing to the regulation of synaptic strength.
Neurotransmitter binding leads to ion flow, resulting in excitatory or inhibitory postsynaptic potentials, which influence the likelihood of an action potential in the postsynaptic neuron.
Neurotransmitter Categories (>100 known)
Acetylcholine (ACh)—formed from acetic acid and choline
Amino acids- triggering various physiological responses: glutamate, aspartate, glycine, \gamma-aminobutyric acid (GABA)
Glutamate: a major excitatory neurotransmitter in the central nervous system, playing a crucial role in synaptic plasticity and memory formation.
aspartate: spinal cord, effects similar to those of glutamate
glycine: inhibitory neurons of the brain, spinal cord, and retina: most common inhibitory neurotransmitter in spinal cord
GABA: a primary inhibitory neurotransmitter in the brain that helps regulate neuronal excitability and plays a vital role in reducing anxiety.
Monoamines (biogenic amines)— synthesized from amino acids by removal of the –COOH group but retain the amino group
Catecholamines: epinephrine, norepinephrine (NE), dopamine
Others: serotonin, histamine
Norepinephrine: a neurotransmitter involved in arousal, attention, and the regulation of mood, which plays a key role in the body's fight-or-flight response. excites cardiac muscle, can excite or inhibit smooth muscle and glands depending on location
epinephrine: hypothalamus, thalamus, spinal cord, and adrenal medulola, effects similar to those of norepinephrine
dopamine: hypothalamus, limbic system, cerebral cortex, and retina, highly concentrated in substantia nigra of midbrain; involved in elevation of mood and control of skeletal muscles
serotonin: a neurotransmitter that stabilizes mood, feelings of well-being, and happiness; primarily found in the brain, intestines, and blood platelets, it plays a crucial role in regulating mood, appetite, sleep, and digestion.
histamine: a neurotransmitter involved in the regulation of sleep-wake cycles, attention, and arousal; primarily found in the brainstem and hypothalamus, it is also implicated in allergic responses and local immune reactions.
Purines: ATP, adenosine
Gases: nitric oxide (NO), carbon monoxide (CO); synthesized as needed rather than stored in vesicles
Neuropeptides: chains of 2-40 amino acids;
cholecystokinin (CCK) and endorphins, substance P, cholecystokinin, enkephalins, endorphins (dense-core vesicles)Stored in large secretory granules (dense-core vesicles)
Some also function as hormones or neuromodulators
substance p: a neuropeptide involved in the transmission of pain and inflammation, often released from sensory neurons and plays a role in the body's pain perception.
Enkephalins: various areas of the brain, spinal cord, act as analgesics by inhibiting substance P, secretion increases during labor
\beta-endorphin: in digestive tract, spinal cord, and many parts of the brain; secreted as a hormone by the pituitary; suppresses pain; reduces fatigue; may produce "runner's high"
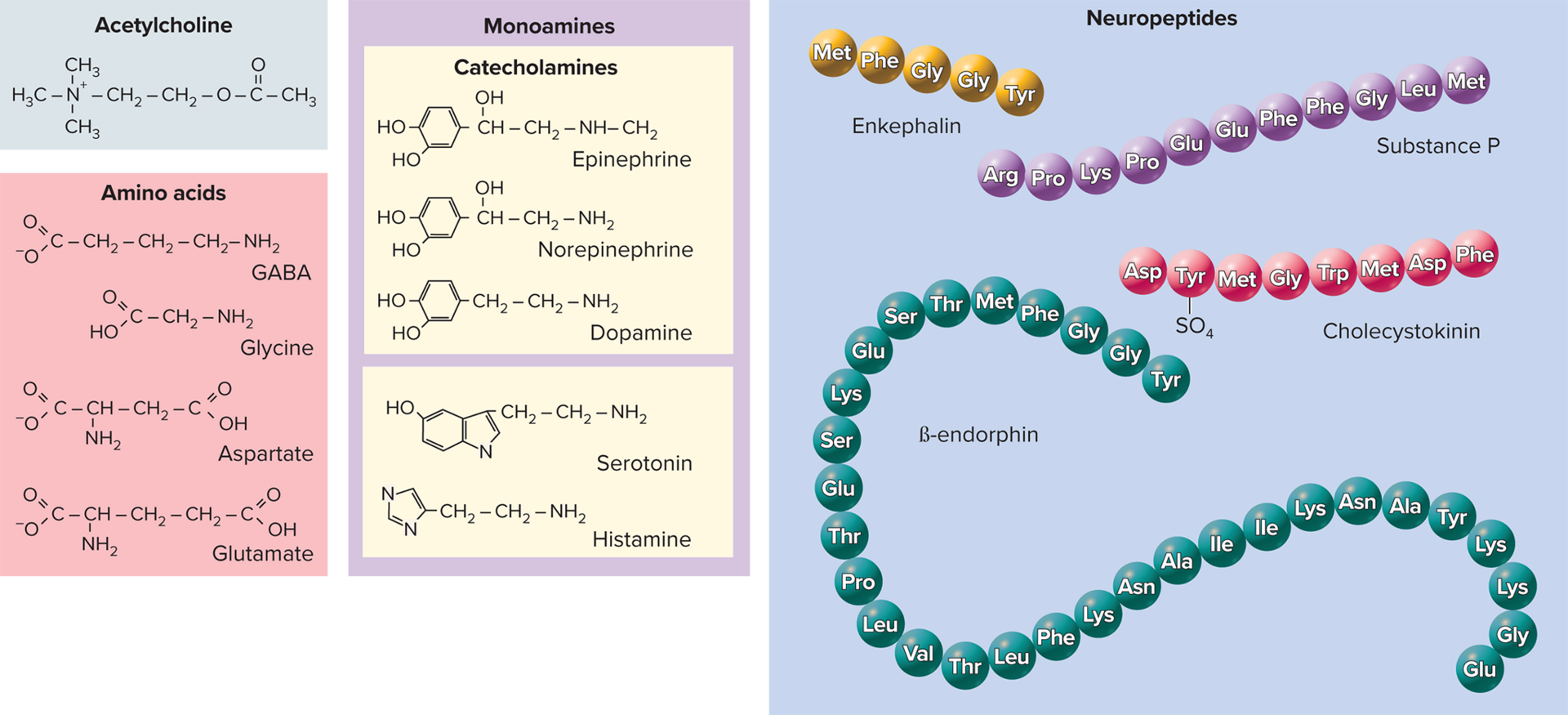
Cholecystokinin (CCK): a peptide hormone produced in the small intestine; stimulates gallbladder contraction, promotes satiety, and mediates the digestion of fats and proteins.
Nociceptin: a neuropeptide that plays a role in pain modulation and is involved in various physiological processes, including appetite regulation and stress response.
Neuromodulator: a type of neurotransmitter that modifies the activity of neurons and synapses, influencing mood, behavior, and cognitive function.
Representative Chemical Synapses
not all neurotransmitters have an excitatory effect
Are the site of learning and memory
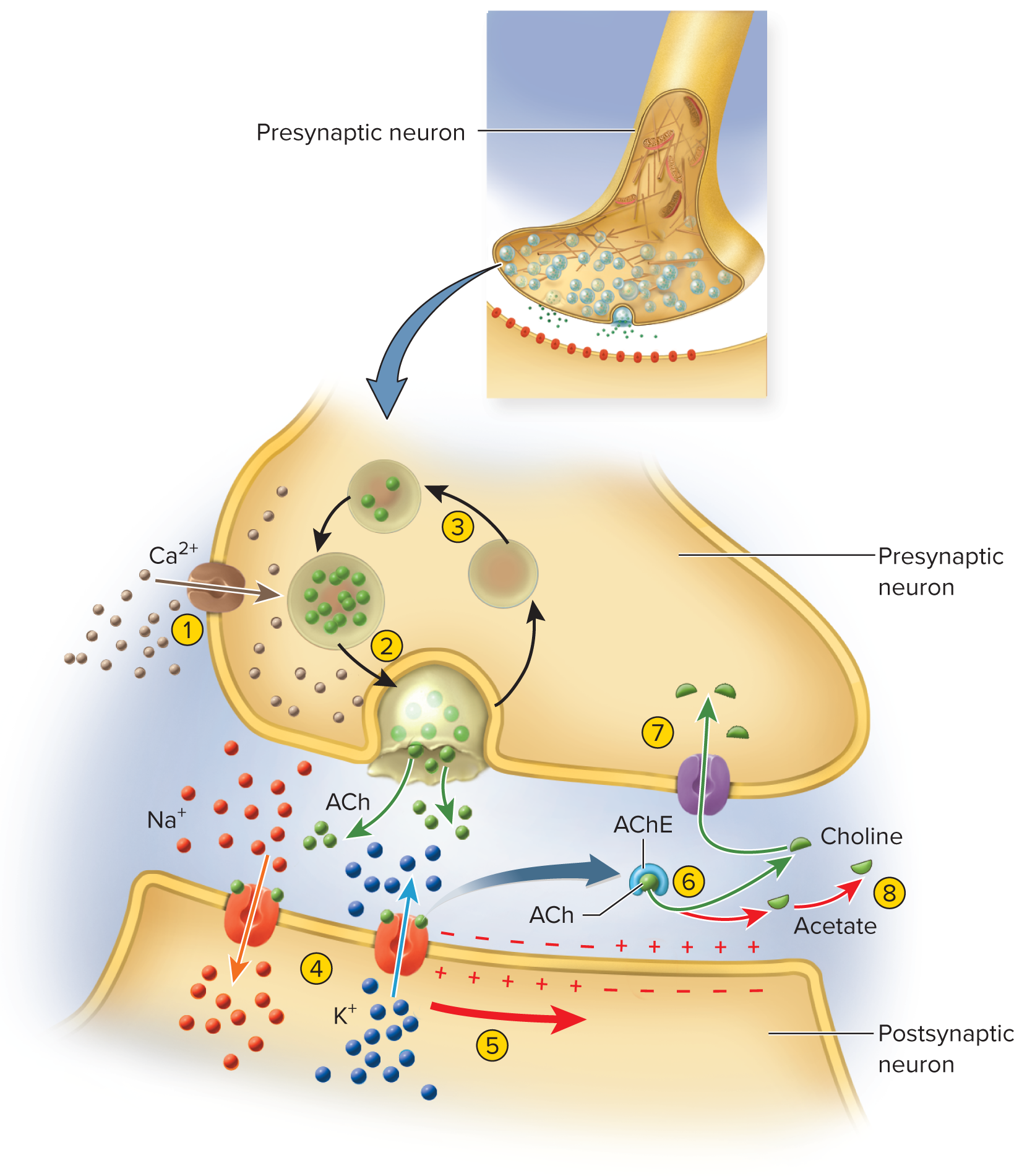
Excitatory cholinergic synapse-(ACh) acetylcholine is the
neurotransmitterAP opens Ca^{2+} channels from depolarization of axon terminal
Ca^{2+} enters, triggers vesicle exocytosis of ACh
ACh diffuses across cleft, binds to postsynaptic receptors
ACh receptors are ligand-gated Na+ channel open allow Na+ and K+ → EPSP membrane
Entry of Na+ causes depolarizing postsynaptic potential
An Inhibitory GABA-ergic synapse
synapses that employ GABA as their neurotransmitter are called GABA-ergic synapses, which typically result in hyperpolarization of the postsynaptic membrane, making it less likely for an action potential to occur.
GABA opens ion Cl^- channels ⇒ hyperpolarization (IPSP less likely to fire action potential)
GABA-ergic synapse—γ-aminobutyric acid (GABA) is
the neurotransmitterpostsynaptic receptor for the GABA neurotransmitter is a potassium channel
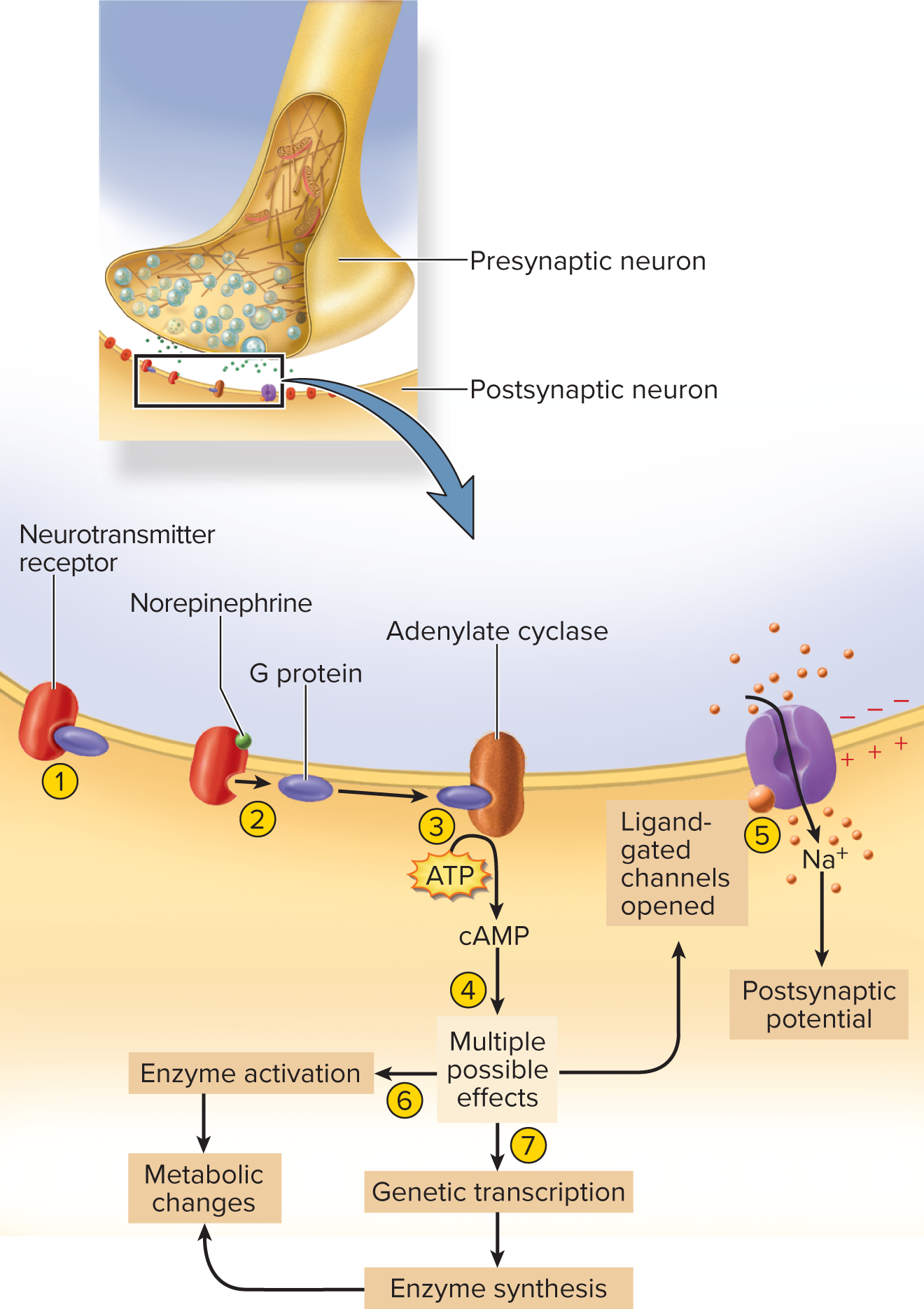
Excitatory adrenergic (NE) Adrenergic synapse—norepinephrine (NE) is the neurotransmitter
G-protein coupled; activates cAMP second messenger → enzyme amplification, slower but powerful
Monoamines and neuropeptides bind to G-protein coupled
receptors on postsynaptic membraneActivate second-messenger systems such as cyclic AMP
(cAMP)Slower to respond than cholinergic and GABA-ergic synapses
Has advantage of enzyme amplification
adrenergic synapse:
employs the neurotransmitter norepinephrine, facilitating the activation of specific receptors that lead to prolonged responses in target cells.
NE, other monoamines, and neuropeptides acts through second messengers such as cyclic AMP (cAMP)
the receptor is a transmembrane protein associated with G protein on the inner face of the membrane
1. the unstimulated NE receptor is bound to a G protein
2. binding of NE to the receptor causes the G protein to dissociate from it
3. The G protein binds to adenylate cyclase and activates this enzyme, which converts ATP to cAMP
4. cyclic AMP can induce several alternative effects in the cell
5. one effect is to produce an internal chemical that binds to a ligant-gated ion channel from the inside, opening the channel and depolarizing the cell ATP is converted to cyclic AMP by adenylate cyclase
6. Adenylate cyclase is activated, another is to activate preexisting cytoplasmic enzymes, which can lead to diverse metabolic changes (indusing a liver cell to break down glycogen and release glucose into the blood)
7. yet another is for cAMP to induce genetic transcription, so that the cell produces new enzymes leading to diverse metabolic effects
GABA-ergic synapses, adrenergic synapses do have signal amplification
a single NE molecule binding to a receptor can induce the formation of many cAMP’s. each of those can activate many enzyme molecules or induce the transcription of a gene to generate numerous mRNA molecules
each can result in production of a vast number of enzyme molecules and metabolic products such as glucose molecules
synaptic events require 0.5 ms or so with an interval called synaptic delay
the time from the arrival of a signal at the axon terminal of a presynaptic cell to the beginning of an action potential in the postsynaptic cell
Signal Termination (cessation of the signal)
Stop transmitter release & clear cleft via:
Enzymatic degradation (e.g., AChE)
Reuptake (e.g., NE, amino acids) → MAO degrades in terminal
Diffusion away → astrocytes uptake in CNS
Neurotransmitter degradation—enzyme in synaptic cleft breaks down neurotransmitter
Example: acetylcholinesterase (AChE) breaks ACh down into choline and acetate
Reuptake—neurotransmitter or its breakdown products reabsorbed into axon terminal
Amino acids and monoamines also reabsorbed, degraded in axon terminal by enzyme monoamine oxidase (MAO)—target of some antidepressant drugs
Diffusion—neurotransmitter or its breakdown products simply away from synapse into nearby ECF
astrocytes-
Neuromodulation
chemicals secreted by neurons that have long term effects on groups of neurons
they adjust or modulate activity of neuron groups in various ways, increasing release of neurotransmitters by presynaptic neurons
May alter the rate of neurotransmitter synthesis, release,
reuptake, or breakdown
• May adjust sensitivity of postsynaptic membraneNeuromodulators = long-term adjusters (frequency, synthesis, degradation, receptor sensitivity)
Nitric oxide (diffusible gas) is a simple neuromodulator
Gas that enters postsynaptic cells and activates second messenger pathways (for example, relaxing smooth muscle)
Neuropeptides chains of amino acids that can act as
neuromodulators (endorphins/enkephalins) suppress pain pathwaysEnkephalins and endorphins are neuropeptides that inhibit pain signals in the CNS
Neural Integration—the ability to process, store, and recall
information and use it to make decisions
Chemical synapses allow for decision-making
Brain cells are incredibly well connected, allowing for complex integration
Pyramidal cells of cerebral cortex have about 40,000 contacts with other neurons
Trade off: chemical transmission involves a synaptic delay that makes information travel slower than it would if there was no synapse
Postsynaptic Potentials
Two types of postsynaptic potentials produced by
neurotransmitters:
EPSP (excitatory postsynaptic potential): Depolarizing voltage
change from the RMP toward threshold. ; usually results from Na+ flowing into cellIPSP inhibitory postsynaptic potential: hyperpolarizing;
voltage becomes more negative than it is at restIPSP can result from K+ exit from cell or sodium Cl^{-} entry
Neurotransmitter effect depends on receptor subtype (e.g., ACh excitatory on skeletal muscle, inhibitory on cardiac muscle)
Different neurotransmitters cause different types of postsynaptic potentials
Glutamate and aspartate produce EPSPs in brain cells
Glycine and GABA produce IPSPs
A neurotransmitter might excite some cells and inhibit others,
depending on the type of receptors in the postsynaptic membraneAcetylcholine (ACh) and norepinephrine work this way
ACh excites skeletal muscle but inhibits cardiac muscle
due to the expression of different types of ACh receptors
on the different types of muscle cells
Summation & Modulation
Summation—the process of adding up postsynaptic
potentials and responding to their net effect
Occurs in the trigger zone
Some incoming nerve fibers may produce EPSPs while
others produce IPSPsA neuron’s response depends on whether the net input is
excitatory or inhibitoryThe balance between EPSPs and IPSPs enables the
nervous system to make decisions
Two ways EPSPs can be added to reach threshold:
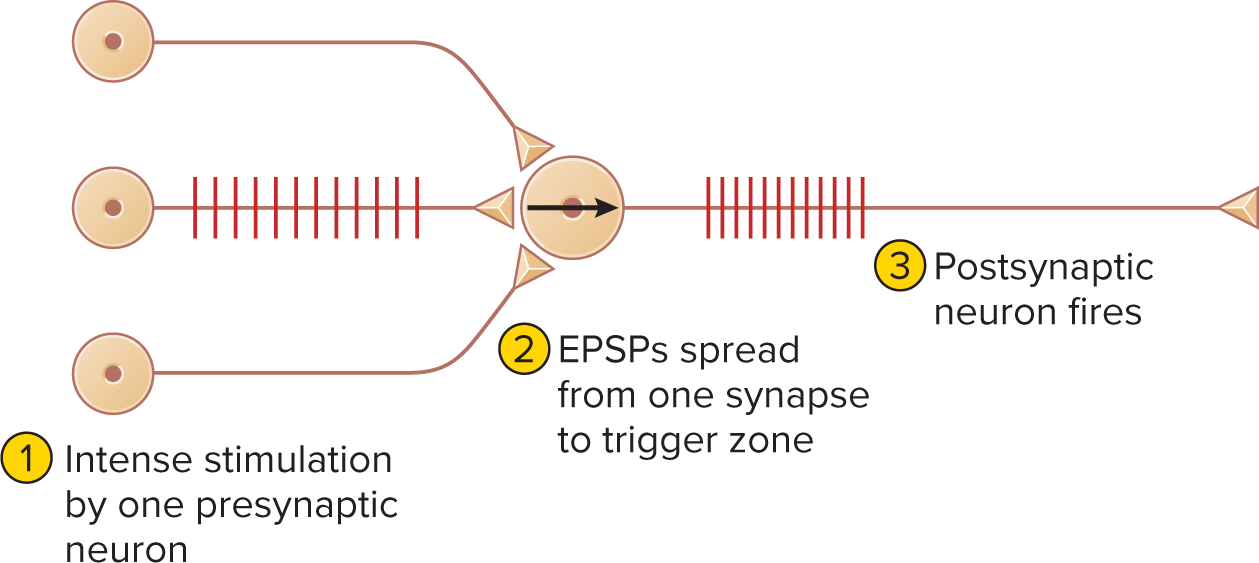
Temporal summation: rapid EPSP succession at one synapse.
a single synapse generates EPSPs so quickly that each is generated before the previous one fades
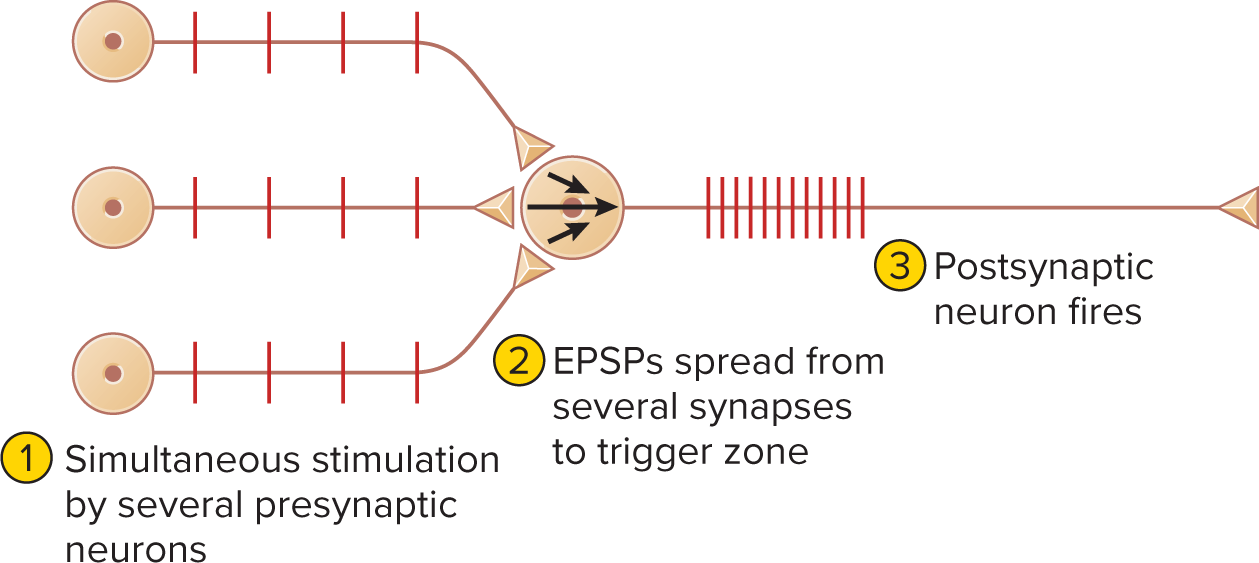
Spatial summation: simultaneous EPSPs at multiple synapses add up to threshold at an axon hillock
presynaptic Facilitation: one neuron (F) enhances another (S) via serotonin prolonging Ca^{2+} influx ⇒ ↑ NT release
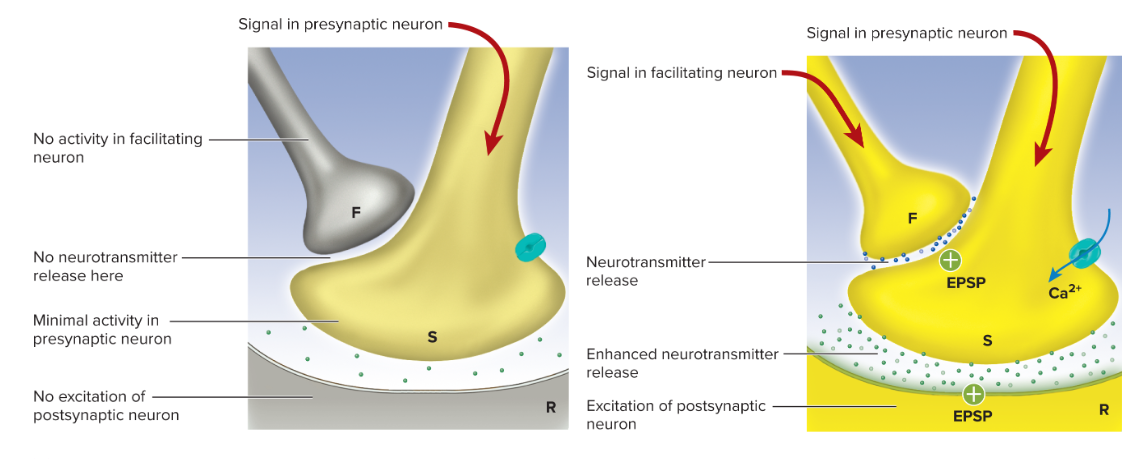
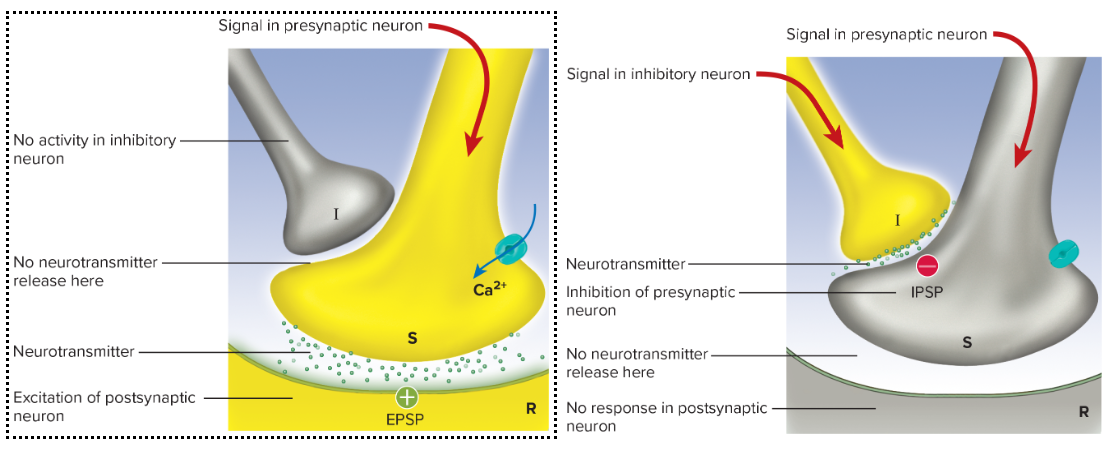
Presynaptic inhibition: is the opposite of facilitation neuron (I) releases GABA onto synaptic terminal ⇒ ↓ Ca^{2+} entry ⇒ ↓ NT release
occurs when one presynaptic neuron suppresses another one
Neural Coding
Qualitative info: “labeled-line” identity of active nerve (optic = light, etc.)
Quantitative info (intensity):
Recruitment: more neurons (incl. high threshold) fire
Firing frequency: stronger stimulus ⇒ higher Hz
Neural Pools & Circuits
Discharge vs facilitated zones (input neuron influence gradient)
Circuit motifs
Diverging, converging, reverberating (oscillatory), parallel after-discharge (after-effect)
diverging: a circuit motif where one input neuron branches to activate multiple output neurons, allowing for widespread signal distribution.
converging: funnel where inputs are channeled into fewer outputs. A circuit motif in which multiple input neurons send signals to a single output neuron, enhancing the strength of the signal and allowing for integration of information from various sources.
reverberating: a circuit motif that involves feedback connections where the output signal re-excites the input neurons, creating a loop that can result in sustained activity and oscillatory patterns in neural signaling. Neurons stimulated each other in a linear sequence from an input to output neurons with some late in the path sending axon branches to neurons earlier in the path
parallel: a circuit motif that allows for simultaneous processing as multiple input neurons connect to multiple output neurons, facilitating an efficient and rapid response to stimuli by distributing signals across various pathways.
serial: a circuit motif where input signals are processed in a sequential manner, with each output neuron passing its signal to the next in line, often resulting in a more linear and straightforward pathway for signal transmission.
Processing styles
Serial: one stream at a time (e.g., reading vs watching TV)
Parallel: simultaneous diverse pathways (e.g., driving integrates visual, auditory, proprioceptive cues)
Memory & Synaptic Plasticity
Engram: anatomical memory trace; basis = plasticity of synapses
Immediate memory: few s; reverberating circuits; enables “present moment” & sentence comprehension
Short-term (working) memory: s → h; tetanic stimulation ⇒ Ca^{2+} buildup ⇒ synaptic facilitation; post-tetanic potentiation retrieves recent memories with minimal cue
Long-term memory
Explicit (declarative): facts, events; conscious recall
Implicit (procedural & emotional): unconscious skills, habits, conditioned responses
Mechanisms: new dendritic spines, larger synapses, long-term potentiation (LTP) via NMDA receptors & Ca^{2+}-dependent signaling
Forgetting: long-term depression (LTD); low frequency → low Ca^{2+} → phosphatases & proteasomes dismantle unused synapses
Degenerative & Demyelinating Disorders
Multiple Sclerosis: CNS demyelination; autoimmune; onset 20–40 yr; neurological deficits ⇒ fatal 25–30 yr post-dx
Tay–Sachs: GM2 ganglioside accumulation; Eastern-European Jewish infants; blindness, dementia; fatal < 4 yr
Gliomas / Brain tumors: arise from mitotic glia; BBB limits chemo ⇒ surgery/radiation
Alzheimer Disease
\beta-amyloid plaques, neurofibrillary tangles; cortex atrophy
ACh & NGF deficits; 11 % >65 affected; research seeks plaque clearance
Parkinson Disease
Dopaminergic neuron degeneration (substantia nigra) ⇒ basal nuclei overactivity
Resting tremor, bradykinesia, pill-rolling; treated with L-dopa, MAO-B inhibitors, surgery
Conduction Velocity Examples (Numeric Recap)
Small unmyelinated: 0.5\text{–}2.0\;\text{m/s}
Small myelinated: 3\text{–}15\;\text{m/s}
Large myelinated: \le 120\;\text{m/s}
Key Equations & Numeric Facts
Resting membrane potential: V_{\text{rest}} \approx -70\,\text{mV}
Threshold voltage: V_{\text{th}} \approx -55\,\text{mV}
AP peak: +35\,\text{mV}
Na\textsuperscript{+}/K\textsuperscript{+} pump: 3\,\text{Na}^+ \rightarrow \text{ECF},\; 2\,\text{K}^+ \leftarrow \text{ICF} + ATP hydrolysis
Fast axonal transport: 200\text{–}400\,\text{mm/day}; Slow: 0.2\text{–}0.5\,\text{mm/day}
Schwann cell myelin layers: up to \sim 100$$ wraps
End of comprehensive bullet-point notes for Chapter 12: Nervous Tissue.