Chemical Bonds
Ionic Bonding
Formation of Ions
Ions are formed when atoms gain or lose electrons
Isoelectronic = Ions that have the same number of electrons as each other
Trends in Ionic Size
Positive ions are smaller than their corresponding atoms, vice versa for negative ions
{Because outer electron is an energy level closer to the nucleus, so less shielding, and greater attraction from nucleus to outer shell electrons}
{For negative ions, electrons enter same energy level, so slightly less attraction from nucleus and therefore the electron is held further away}
Ionic radii increase in size down a group
{As you go down a group, the outer electron is held in an energy level further from the nucleus, so greater shielding and weaker attraction to outer electrons}
As charge on the ion increases, the ionic size decreases
{As proton number increases there is a greater attraction from nucleus to outer electron and same shielding}
All this is because fewer electrons so attraction from nucleus to outer electron is strong
Structure of Ionic Compounds
Highly ordered giant lattice consisting of oppositely charged ions held together by strong electrostatic forces of attraction.
Melting and boiling points of ionic compounds are high
MgO has a higher melting point than NaCl, because the ions (Mg2+ and O2-) are smaller, and more highly charged than NaCl.
They are generally brittle as any movement of the planes of ions results in similarly charged ions being opposite one another, causing the planes to repel each other.
They conduct electricity when molten or dissolved in water; ions are free to move. They are often soluble in water.
Covalent Bonding
Formation of Covalent Bonds
It is a shared pair of electrons between 2 atoms
Co-Ordinate Bonding (Dative Covalent)
Both of the electrons in the shared pair originate from one of the atoms.
Melting and Boiling Point of Covalent Molecules
Low melting points due to weak intermolecular forces. They are not soluble in water and do not conduct electricity.
Bond Polarity in Covalent Molecules
Non-Polar = bonding electrons are shared equally; no charges on atoms OR bonding electrons are shared unequally, but the shape of the molecule means there is no positive or negative end. They are symmetrical; electron density equally distributed.
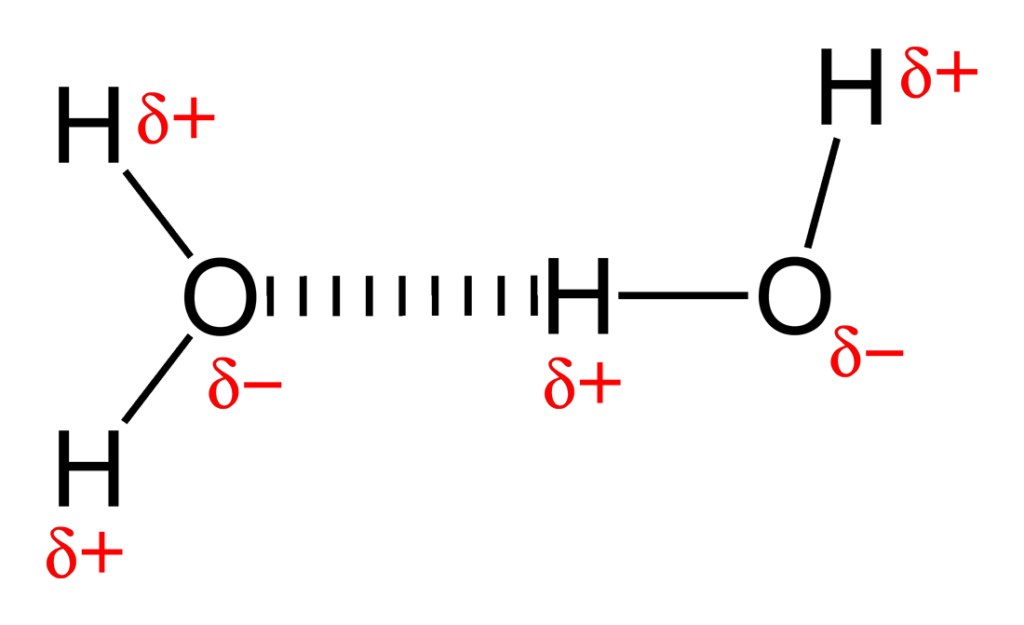
A polar bond is a type of covalent bond between two atoms where there is a significant difference in electronegativity, resulting in an uneven distribution of electron density. This causes one end of the bond to be slightly negative (the more electronegative atom) and the other end to be slightly positive (the less electronegative atom).
For example, in a water molecule (H₂O), the oxygen atom is more electronegative than the hydrogen atoms, leading to a polar bond where the oxygen atom carries a partial negative charge (δ-) and the hydrogen atoms carry partial positive charges (δ+).
Electronegativity
The power of an atom to attract a pair of electrons in a covalent bond to itself.
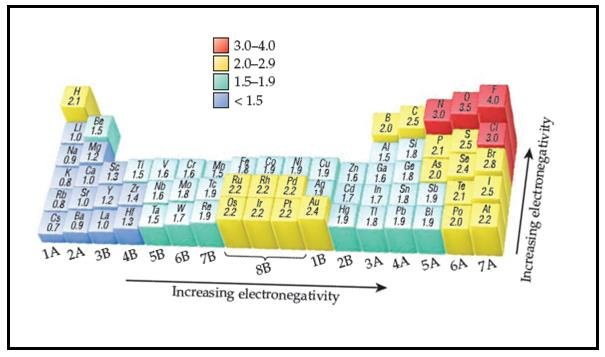
Across a period, there is an increase in electron attracting power. This is because of the decrease in atomic radius of the atoms across a period, increasing the attraction of the nucleus on outer electrons.
{Increase number of protons, similar shielding}
Group 1 metals possess low electronegativity values because they lose their outer electrons very easily.
Large difference in electronegativity of elements = Ionic bonding
Small difference in electronegativity of elements = Covalent bonding
2 metals = Metallic bonding
Forces of Attraction: Between Covalently-Bonded Molecules
Forces of attraction between simple covalent molecules are weak. This means they have low melting and boiling points as little energy is needed to overcome these forces.
Covalent bonds > Hydrogen bonds > Van der Waals
Van der Waals (VdW)
Van der Waals forces are weak intermolecular attractions that occur between molecules or between different parts of a single molecule. These forces arise from temporary fluctuations in electron distribution within molecules, leading to the formation of temporary dipoles.
Occur due to the momentary distribution of electrons in atoms and nonpolar molecules, resulting in temporary dipoles. These forces increase with the size of the molecules or atoms, as larger atoms have more electrons and a greater chance of creating temporary dipoles.
Because they are weak, they require little energy to be overcome. Molecules with just van der Waals have low melting and boiling points.
Branching of the C chain leads to smaller intermolecular forces as the molecules are smaller and more spherical, so lower S.A, so less movement of electron density and weaker VdW forces between molecules.
Permanent Dipole-Dipole
Polarity of Molecules: For permanent dipole-dipole interactions to occur, the molecules must be polar, meaning they have a region of partial positive charge and a region of partial negative charge. An example is hydrogen chloride (HCl), where the chlorine atom is more electronegative than hydrogen, resulting in a permanent dipole.
Attraction Between Dipoles: The positive end of one polar molecule is attracted to the negative end of another polar molecule. This attraction can significantly influence the physical properties of substances, such as boiling and melting points.
Strength of Interactions: While permanent dipole-dipole interactions are stronger than London dispersion forces, they are generally weaker than hydrogen bonds. Their strength can vary based on the specific molecules involved and the distance between them.
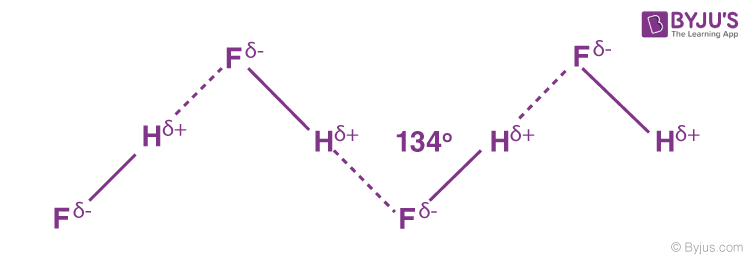
Hydrogen Bonding
Hydrogen bonding is a type of strong intermolecular force that occurs when hydrogen is covalently bonded to a highly electronegative atom, such as nitrogen (N), oxygen (O), or fluorine (F). This creates a significant dipole in the bond due to the large difference in electronegativity between hydrogen and the electronegative atom. The positively charged hydrogen atom can then attract the lone pair of electrons on another electronegative atom from a nearby molecule, creating a hydrogen bond.
Higher Boiling and Melting Points: Substances capable of hydrogen bonding exhibit higher boiling and melting points compared to similar molecular weight compounds that cannot form hydrogen bonds.
Solubility: Polar molecules that can hydrogen bond are often soluble in water, while nonpolar substances are not
Metallic Bonding
Ions in a regular lattice arrangement
Sea of delocalised electrons
Electrostatic force of attraction between positive metal ions and delocalised electrons
Delocalised electrons are free to move and carry a charge around the whole structure.
In group I metals, the melting point decreases as metal ions get larger and shielding increases. Weaker electrostatic attraction from nucleus to the delocalised electrons
Strength of Metallic Bonds
Strength increases and melting point of a metal increase as the size of the positive ion decreases and as the number of outer electrons increases
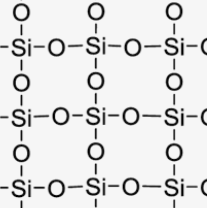
Giant Covalent Lattice (Macromolecular)
Diamond
Each C is surrounded by 4 other C atoms linked tetrahedrally to 4 atoms. Diamond is hard and has a very high mp and bp.
High Melting/Boiling Point
Strong covalent bonds that require lots of energy to break
Does Not Conduct Electricity
No delocalised electrons or free ions to move and carry a charge
Graphite
Graphite is a layered structure. Each C is bonded to 3 other C atoms. Weak VdWs holding the layers together.
High Melting Point
Strong covalent bonds between atoms in each layer require a lot of energy to break
Conducts Electricity
The last electron is released and becomes delocalised; free to move through the structure and carry a charge
Brittle / Lubricant
Weak VdWs between layers. Easy to break, so layers slide over each other easily.
States of Matter
Solid - Greater vibration of particles at a fixed point.
Liquid - Faster and more freely
Gas - Rapid, random movement
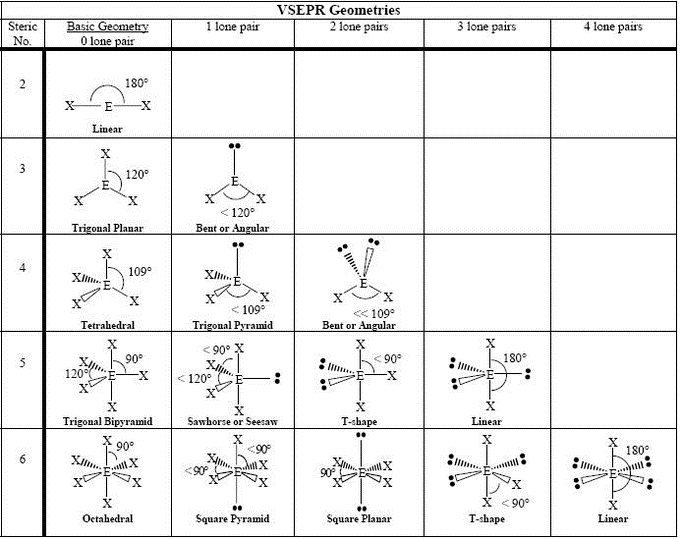
Shape of Simple Molecules and Ions
Number of electron pairs = (number of outer electrons in the central atom + number of simple covalent bonds formed by the central atom ± charge) / 2