Phagocytes and Granulocytes
3: Derived from myeloid progenitor cells. Macrophages and neutrophils are phagocytes. Neutrophils also carry out degranulation as they contain cytolytic granules and enzyme pathways which assist in elimination of pathogenic microbes. Dendritic cells also phagocytes but more as antigen presenting cells than cell killers.
5: Macrophages are found in every tissue, sentinel cells (patrol) which respond to tissue injury and pathogen invasion. They are distributed in most tissues.
During embryonic development monocytes are dispersed throughout the developing tissue. Differentiated, tissue resident macrophages are present in the tissue from birth.
During macrophage maturation, bone-derived monocytes move into the blood and migrate to tissue, responding to migratory and differentiation signals along the way.
6: Macrophages are terminally differentiated but highly plastic - can change phenotype - functions change in response to environmental changes. M1 produce pro-inflammatory cytokines and chemokines and promote adaptive immunity.
M2, or ‘alternatively activated’, helps dampen down and resolve inflammation for tissue repair and remodelling by anti-inflammatory cytokines, chemokines and growth factors. Full spectrum of different phases of activation of macrophages, not just M1 and M2.
7: 40 to 70% of circulating white blood cells in humans are neutrophils. They have a multilobulated nucleus and a very short lifespan of 5 to 135 hours - constantly being replenished but only when needed. Neutropenia makes an individual highly susceptible to infection - common in chemo and AIDS patients.
8: Immune response through macrophages and neutrophils:
Tissue macrophage senses infection
Macrophages mount immune response and immune cells recruited via chemokines and cytokines eg granulocyte-macrophage-colony stimulating factor (GM-CSF)
Neutrophils released bone marrow → circulation, migrate to infection site
monocytes infiltrate tissues, differentiate and remove dead cells for tissue repair (resolution)
9: Neutrophils not normally found in healthy tissue - need recruitment. sense and respond to soluble inflammatory signals. Recruitment of neutrophils regulated by adhesion molecules between leukocytes and the inflamed endothelium. This is followed by extravasation (leakage) → local tissues via chemotaxis.
Classical neutrophil recruitment cascade:
capturing (tethering)
rolling
slow rolling
arrest
transmigration
10: cytokines upregulate presentation of selectins on endothelial cells
neutrophils need to cross basement membrane to enter infected tissue - may need to use proteases to break up extracellular matrix proteins
11:
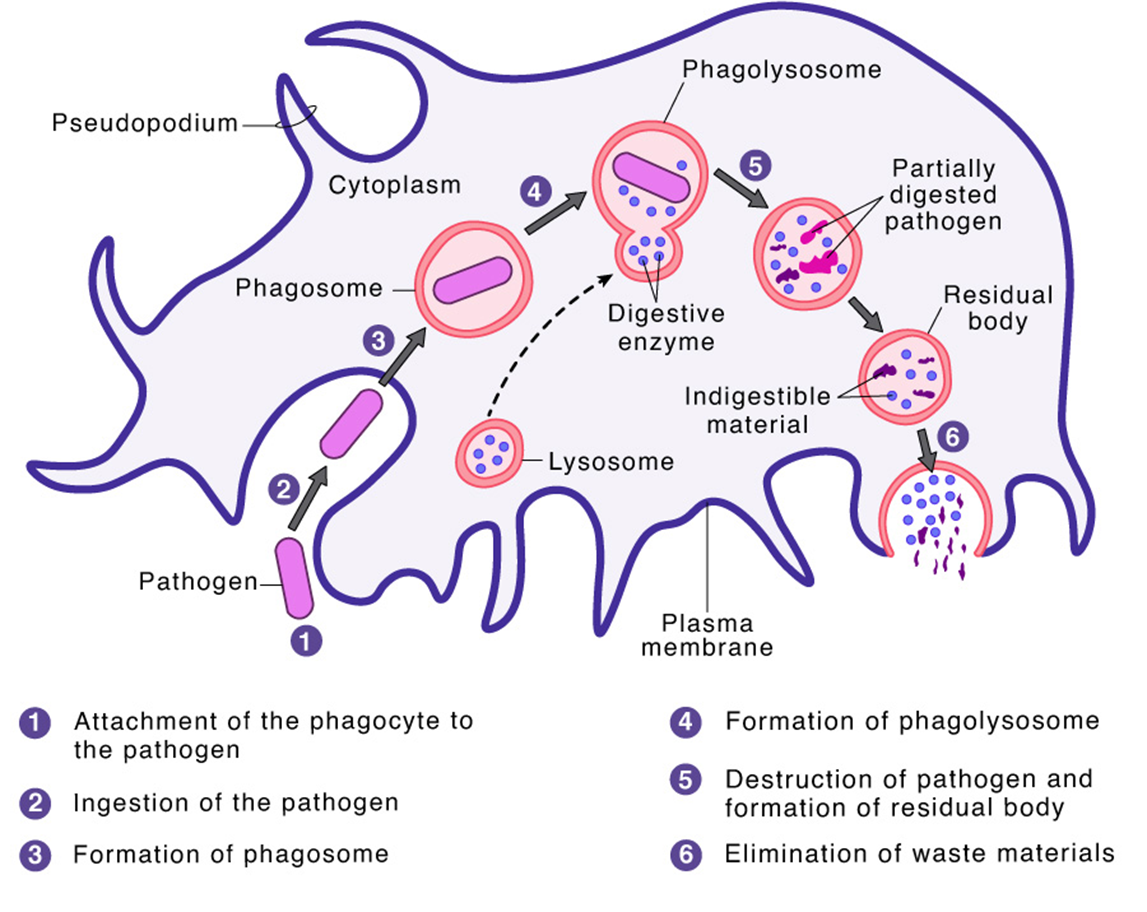
12: Phagocytosis is the engulfing of large particles (greater than 0.5 micrometres) by eukaryotic cells.
Recognition of invading microbe → ingestion, phagosome formation → phagolysosome formation → microbial killing, residual body forms → elimination/exocytosis
image shown = healthy and smoker phagocyte
13: Recognition:
Innate immune cells recognise pathogens via PAMPs recognised by PRRs. TLRs aren’t phagocytic receptors but work with phagocytic receptors to make phagocytosis more efficient.
Receptors which bind PAMPs directly and induce phagocytosis inc glucan receptors (Dectin-1) and mannose receptor.
14: Recognition:
Opsonins are soluble molecules deposited onto foreign surfaces. Act as adaptors which bind and activate phagocytic receptors. Examples include antibodies, fibronectin and complement factors.
FcRs and complement receptors are the best characterised opsonic receptors. FcRs bind Fc of IgG and IgA. Complement receptors bind activated complement components eg iC3b.
During phagocytosis, integrins cooperate with Fc gamma receptors by promoting adhesion to opsonised particle. Aggregation of Fc gamma receptor triggers inside-out signal which activates integrins via GTPase Rap (?). The activated integrins also bind the particle via complement fragment C3b.
15: Ingestion involves complex signalling pathways causing cytoskeleton remodelling, dynamic membrane movement and fission.
16: pseudopodium envelopes pathogens
17: Autophagy, or ‘self eating’ is the delivery of cytoplasmic proteins and organelles to the lysozyme for degradation. It’s activated in response to various stress conditions eg starvation, and can be selective or non-selective. Similar process to phagocytosis but for very different reasons.
Non selective = part of cytoplasm engulfed due to amino acid deprivation
Selective = xenophagy (degradation of microbes)
Autophagy involves double-membrane compartments forming around target bacteria, and their transport to lysosomes. Autophagy-related genes (ATGs) coordinate engulfing and degradation of target cargo. LC3 facilitates lysosome binding phagosome.
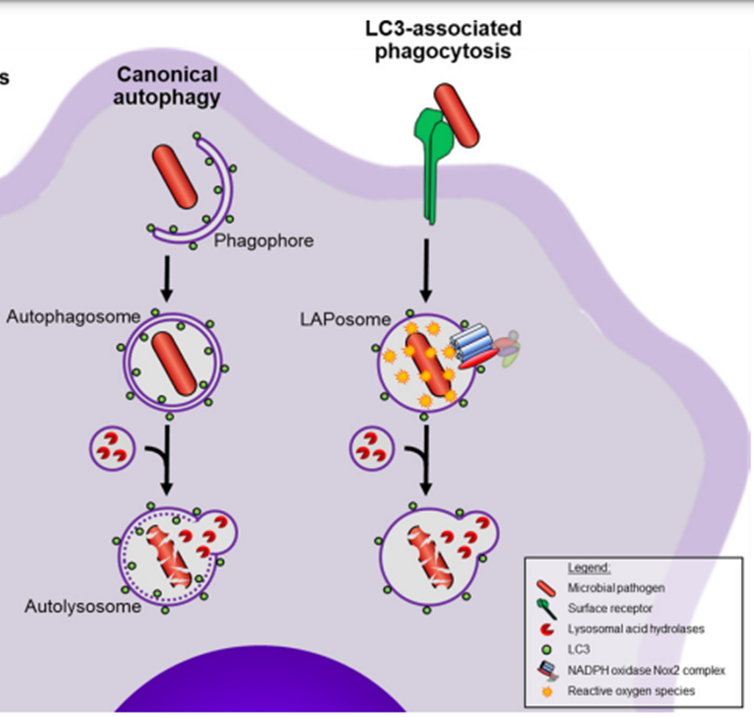
18: Phagosomes fuse lysosome = phagolysosome. Microbial killing then achieved by non-oxidative and oxidative mechanisms.
Oxidative killing | Non-oxidative killing |
superoxide anion | lysozyme |
hydroxyl radicals | hydrolytic enzymes |
hypochloride anion | transferrin |
nitric oxide | defensins |
nitrogen dioxide | |
nitrous acid | |
NETosis |
19: Respiratory bursts = major way of killing in phagolysosome. Non-mitochondrial generation of antimicrobial reactive oxygen species through NADPH oxidase enzyme (NOX) complex.
NOXs are transmembrane enzymes which specifically produce cytosolic ROS. Made up of gp91 and p22 subunits forming cytochrome b558, and several cytosolic components eg Rac1 protein.
When phagocyte is activated the cytosolic components and cytochrome b558 assemble in the cytoplasmic membrane.
20: NOXs transfer one electron from NADPH to oxygen, forming superoxide which is released into the phagolysosome.
Chronic granulomatous disease (CGD) is a genetic disorder characterised by mutations in genes encoding NOX2 complex components. CGD patients are hypersensitive to bacterial and fungal infections as their phagocytic cells can’t kill pathogens due to very low oxidative burst.
21: Superoxide has a low bacteriocidal potency and thus is converted into H2O2 which may react with superoxide to generate hydroxy radicals which are highly reactive and toxic.
22: Myeloperoxidase is present in neutrophils and reacts with H2O2 and chloride → hypochlorous acidhypochlorite (HOCl). Target chlorination inactivates proteins and enzymes, can interfere with bacterial replication inside phagolysosome.
23: Reactive nitrogen species: Macrophage inactivation → inducible nitric oxide synthase expression (iNOS). iNOS oxidises L-arginine, generating nitric oxide. Superoxide and NO can react, forming peroxynitrite (ONOO-, v reactive).
NO balance competitively regulated as Th2 cytokines inhibit NO production eg IL4 or IL13. Th1 cytokines enhance NO production.
24: Hydrolytic enzymes (eg nucleases, proteases, glycosidases, lipases, phosphatases, sulfases and phospholipases) are stored in lysozymes and work at optimal pH 4.5-5. pH is maintained by H+ ATPase (hydrogen pump).
Hydrolytic enzyme action produces antigenic peptides for class II MHC loading and make a significant contribution to cell killing
25: Neutrophils contain 4 key types of granules which are formed in neutrophil differentiation. Listed below are the 4 types and their major constituents
azurophil
Antibacterial proteins: myeloperoxidase and CAP37. Proteases: neutrophil elastase, cathepsin G and proteinase-3
specific
Antibacterial proteins: lactoferrin, neutrophil gelatinase-associated lipocalin, cathelicidin and lysozyme
Proteases: collagenase
gelatinase
Antibacterial proteins: lysozyme
Proteases: matrix metalloproteinase (MMP9) and leukolysin (MMP25)
secretory
transmembrane receptors eg TNF and IFN-alpha receptors. DON’T contain antibacterial proteins or proteases
26: Constituent proteins in neutrophil granules are pre-packaged and readily available for release upon activation. Secretory granule contents are quickest to undergo exocytosis, followed by release of gelatinase granule contents, then specific.
Azurophil granules undergo limited exocytosis and function primarily within phagolysosomes. The proteases degrade extracellular matrix proteins, facilitate immune receptor activation/inactivation, and assist in pathogen digestion and clearance.
27: Certain pathogens have evolved ways to avoid being killed in phagolysosomes:
Staphylococcus aureus and Yersinia species avoid detection by changing structure and recognition of PAMPs and prevent uptake
Mycobacteria, Legionella and Salmonella remodel phagosomes eg halt maturation into phagolysosomes - TB replicate easily inside macrophages and inhibit fusion of phagosome with lysosome
Listeria monocytogenes, Shigella flexneri and Burkholderia pseudomallei destroy phagosome before it fuses with lysosomes
28: NETosis is the formation of neutrophil extracellular traps. Neutrophil specific cell death method characterised by release of large weblike structures. Not dependent on phagocytosis. These structures are made up of modified chromatin (from DNA) decorated with bactericidal proteins from granules and the cytoplasm. NETosis induction is ROS dependent. NETosis destructive to cell but there’s also a more minor form which only releases small DNA amounts so enough retained for cell itself.
29:
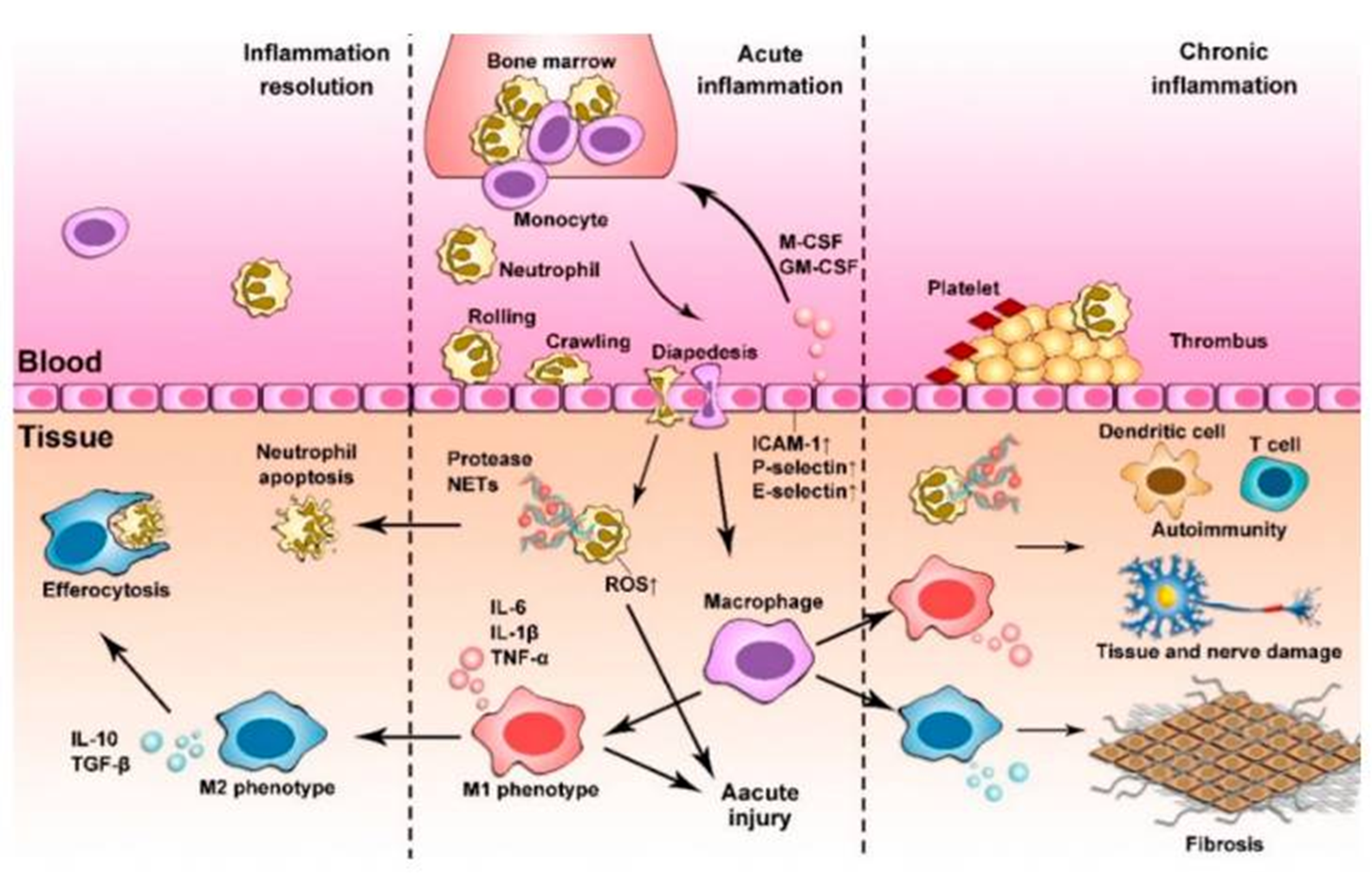
Extra reading REQUIRED:
phagocytosis recognition
phases of phagocytosis
cytosolic burst
key steps in processes and key driver/effector molecules