Chemicals of Life(in depth)
Carbohydrates
Elements present- C, H and O. Preset in a %%C1H2O1%% ratio.
Function- %%Energy and structure.%%
3 different types
- Monosaccharides (simple sugars). The building blocks.
Eg. Glucose, Ribosome, Fructose, Galactose.
Monosaccharides are %%distinguished by the carbonyl group(aldehyde or ketone) and the number of atoms%% in the carbon backbone.
Carbohydrate configurations can be drawn in 2 ways. Chain form and ring form. %%The chain form shows functional units better but the ring form is more accurate to shape.%% The ring is the only shape normally found in biology.
Eg. A glucose molecule.
%%A 6 sided ring is called a pyranose ring.%% And the OH group on C#1 can be found above or below the midline of the ring. %%If it is below it is an alpha glucose and if it is above it is called a beta glucose. 50% become alpha and 50% become beta.%%
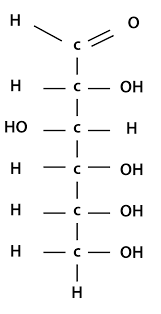
Chain form of glucose.
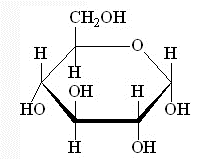
Ring form of glucose.
%%Fructose, galactose ad mannose are all examples of isomers to glucose.%%
Note: an isomer is a molecule that shares the same chemical elements but different structure.
Eg. Ribose and Fructose.
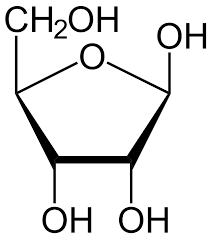
Ribose.
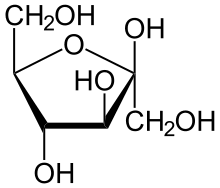
Fructose
- Disaccharides
Disaccharides are more complex sugars. Simple sugars %%joined together with condensation and ether links%%
Eg. Maltose= glucose+glucose
Linkages are either %%1,4 glycosidic or 1,6.%%
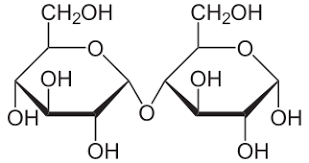
%%The carbon 1 of on molecule is connected to the carbon 4 of the other.%%
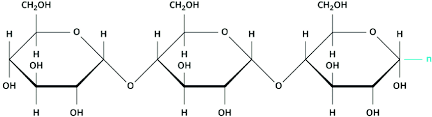
- Polysaccharides
A polysaccharide is when %%many simple sugars join together using 1,4 or 1,6 ether links.%%
Note: Oligosaccharides: smaller polysaccharides (3-10 monosaccharides)
Eg1. Starch
Starch is a %%storage molecules in plants%%. %%It stores glucose%%. It is made up of 2 smaller polysaccharides.
It is %%25% amylose%%- a glucose linked using 1,4 linkages to get a coiled chain. And %%75% amylopectin%%- glucose linked using 1,4 and 1,6 linkages that form tangled short branches.
The more branches the starches are, the harder they are to digest.
Eg2. Cellulose.
%%Main component of plant cell walls%%. Made by joining %%3000+ glucoses w Beta 1,4 links%%.
We have no enzymes that can digest this linkage. And the %%straight unbranched shape allows hydroxyl groups of parallel molecules to form hydrogen bonds and produce tight bundles or fibres.%%
Eg3. Glycogen (animal starch)
This form of %%glucose is store in live/muscle.%% It is shaped like %%amylopectin but even more 1,6 links%% so more branched.
Eg4. Chitin
A %%special polysaccharide%% found on the coverings of insects and crustaceans. It is %%not a true polysaccharide due to the nitrogen.%%
Eg5. Blood groups (A,B,AB,O)
Different blood types cause by the 2 different polysaccharides attached to the membrane of a RBC(red blood cell).
Lipids
Elements present- C,H,O and P(sometimes).
Functions- energy, structure(cell membranes), regulatory jobs(hormones,steroids,insulation, shock absorber)
Types of Lipids
- Fats
%%Most common energy storage.%% They are energy storage efficient because they %%are compact and lightweight. Made of a long chain of fatty acids joined to a glycerol with an ester bonds.%%
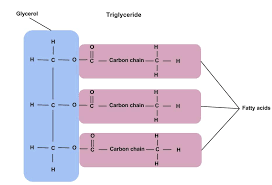
Glycerol is the hydroxyl and fatty acids are carboxyl. Usually contain %%even amounts of carbons%%(14,16,18) and %%may be saturated or unsaturated.%% A %%saturated fat has only single bonds. A unsaturated fat has double/triple bonds that cause kinks.%%
Different lipids are caused by…
I) types of fatty acids joined to glycerol
II)amount of fatty acids
- 1 F.A = monoglyceride
- 2 F.A= diglyceride
- 3 F.A triglyceride
Results in…
I) Fats contain long fatty acids and are saturated.
II) Oils contain short fatty acids and are unsaturated.
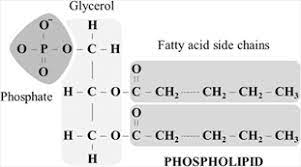
- Phospholipids
%%Lipids by themselves are non-polar and do not like water. BUT phospholipids have one polar end that attracts water and another end that is still non-polar.%% They are made up of glycerol, fatty acids and 1 phosphate containing a functional group.
- Steroids
Compact hydrophobic molecules, %%4 fused hydrocarbon rings and several different functional groups%%.
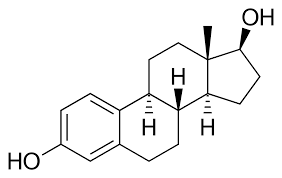
Eg1. Sex hormones- estradiol in female, testosterone in male, they only have a couple small differences.
Eg2. Cholesterol- important in membranes and structure. Cells convert it into vitamin D and bile salts.
- Waxes
Waxes are %%long chains of fatty acids linked to alcohol or carbon rings. Ester links%%.
Eg. Honey comb, earwax, beeswax
Triglyceride (Macromolecules)
%%Main molecules for energy storage. 1 glycerol + 3F.A.%% It is an %%overall non-polar molecules.%% The oxygens do create a small polar section but it does not affect the non-polarness of the molecule.
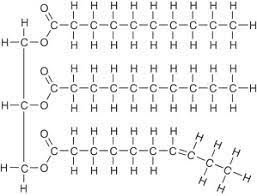
The first 2 fatty acid chains are saturated due to all the carbons being bonded by single bonds. But the bottom chain has a double bond and a kink which makes it unsaturated.
Proteins
Functions:
- %%Structure (muscles, hair, skin, nails, bone)%%
- %%Energy%%
- %%Regulatory (controls enzymes)%%
Structure:
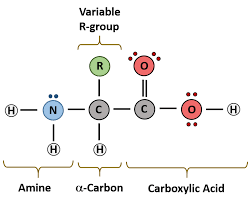
Protein macromolecules- %%amino acids linked together via peptide links.%%
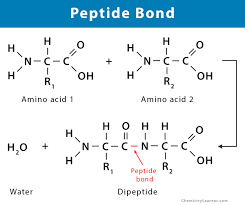
Types of Proteins
Depends on:
- %%amount%% of amino acids (AA) linked together
- %%Type%% of AA linked together (Different side chain combos)
- %%Sequence%% of AA
Protein function is dependant on shape!!!
Protein Structures:
- Primary
- Secondary
- Tertiary
- Quaternary
All proteins have the first 3.
- Primary
%%Number, type and sequence%% of amino acids linked together in a %%chain%%.
All other structures depend on primary.
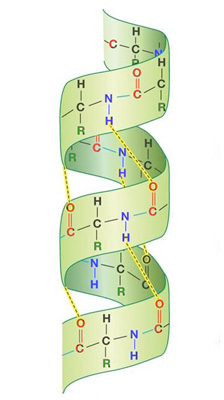
- Secondary
Formed when %%hydrogen bonds are created between amino acids along the chain.%%
2 structures can be formed:
- Alpha-Helix
When the %%electronegative oxygen of 1 peptide link is attracted to an électropositive H of another peptide link.%%
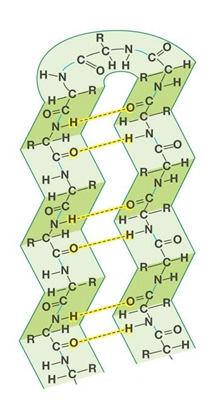
- Beta-sheet
When %%2 peptide chains that lie parallel to each other have H-bonds form between them due to the oxygen of one peptide link attracting to the H of the adjacent peptide link.%%
- Tertiary
Makes the %%overall structure%% of protein. %%Folding created due to R-group interactions.%% Polar R-groups will fold to the outside (hydrophilic) while non-polar R-groups will fold inwards (hydrophobic).
3D shape is stabilized by R-group interactions
- %%H-bonds between R-groups%%
- %%Ionic bonds between charged R-groups%%
- %%Disulphide Bridge between R-groups that contain sulfur%%
- %%Dipole-dipole forces%%
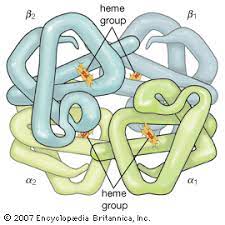
- Quaternary
Not all proteins contain this!
%%Multiple subunits formed together to create a functional protein.%%
Subunit→ polypeptide chain folded into tertiary structure
Eg. Hemoglobin has 4 units (2 alpha, 2 beta)
Nucleic Acids
Functions: cell coordinations- instructions for cell functions
Structure: Nucleotides
- pentose sugar (5 carbon sugar)
Eg. Deoxyribose or Ribose
Inorganic Phosphate (H3PO4)
Nitrogenous Base- nitrogen containing a ring (2 types)
- Pyrimidine(single ring)
Eg. Thymine, Cytosine
- Purines (double ring)
Eg. Adenine, Guanine
Multiple Nucleotides combine to create DNA and RNA (cell information)
Energy Relationship- ATP
Nucleotides provide immediate energy sources for most activities in living cells.
- act as mobile potential energy storage molecules
- Can act as coenzymes- accept and donate e- or h+ in redox reactions
- Adenosine Triphosphate (ATP)
- made of ribose sugar + adenine + 3 phosphate groups (It’s a nucleotide)
- It is formed when ADP + Pi → ATP
- Very acidic due to 4 H protons on phosphate disassociates. Leaves area with -ve charge.
O- is unstable, energetic and holds potential energy.
P Anhydride Linkage
- repulsion of all the -ve charges lead to highly energetic area.
- Represented with a squiggly line.
- Means that a large amount of useful energy is released when the bond is broken by hydrolysis
- The product that results (ADP+Pi) have less free energy than reactants. (ATP+H2O)
Enzymes
- are proteins
- Catalyze chemical reaction
- Like lock and key (fits specific shape)