Alcohol
Muaaz NV2C
Lesson 1:
Distinguish the optical isomers
Notes:
Determine optical isomers:
To figure out the optical isomers you see that a carbon atom has 4 different groups bonded around it.
Why is alcohol liquid:
Hydrogen bonds hold the molecule together and keep it liquid, hence why water is not a gas due to the bonds with hydrogen.
If you can put another molecule closer to the same molecule the closer they are, the more intermolecular forces they have or London dispersion forces (van der Vaal).
Greater branching leads to a lower boiling point as there are fewer interactions and the London dispersion forces are lower than a straight branch
General formula:
Cn
Naming rules:
Select the longest chain of C containing the OH group
Remove the e and add OL after the basic name
Number the chain starting from the end, closer to the O-H group
Same rule as alkenes applies to the naming of the other branches
When two electrons come from the same molecule it’s called a coordinated covalent bond.
You can dehydrate an alcohol to make it an alkene or you can hydrate an alkene to make it an alcohol.
Oxidizing alcohols:
Primary alcohols are easily oxidized to aldehydes
Secondary alcohol can also get oxidized
Tertiary alcohols would not react as it is not possible for a carbon atom to have five bonds around it.
<Todo>
Lesson 2:
Notes:
Go through everything
Notes from the book:
Chapter 13: Alcohols
Alcohols are organic compounds that contain carbon, hydrogen, oxygen, and the hydroxyl function group OH.
To name an alcohol look for the lowest number possible for the hydroxyl group. The hydroxyl group is more important than the position of the double bond or a bond of a halogen atom but it is less important than the carbonyl group (an oxygen atom double bonded to a carbon atom). The presence of multiple hydroxyl groups is written as di, tri, and are written before the suffix ol.
There are two ways of classifying the alcohol. The first one is based on the number of carbons attached to the carbon atom which is bonded with the hydroxyl group. The other way is based on the number of hydroxyl groups present per molecule.
In the first classifying system, there are three types of alcohol.
Primary alcohols are alcohols in which the carbon atom that is bonded to the hydroxyl group is bonded to one carbon atom
Secondary alcohols are carbon atoms bonded to two carbons and a hydroxyl group
Tertiary alcohols are carbon atoms bonded to three carbon atoms and a hydroxyl group
The other system of classifying is based on the number of hydroxyl groups present per molecule where:
Monohydric alcohol has one hydroxyl group.
Dihydric alcohol has two hydroxyl groups.
Trihydric alcohols have three hydroxyl groups.
Polyhydric alcohols have many hydroxyl groups
The boiling point of alcohol is directly proportional to the amount of carbon in the chain. For an alcohol to boil it must conquer two types of intermolecular forces, these forces keep them together. These forces are:
Weak induced dipole-induced, is a dipole force that is present in all molecules as a result of temporary dipoles within the molecule. This force becomes stronger as the molecule becomes larger and contains more electrons. In alcohols, this force is the attraction between the alkyl part of one molecule and the alkyl part of another molecule.
Hydrogen bonds are much stronger. The hydroxyl group in alcohol has a polar covalent bond in which the oxygen is slightly positive and the hydrogen is slightly positive because the oxygen is naturally more electronegative than hydrogen, this results in the molecule having a permanent dipole in which hydrogen is attracted to the oxygen for its negative charge.
The solubility of alcohol in water is inversely proportional to the amount of carbons present in the chain. For a molecule to dissolve in water it must be able to interact with the water molecules (H2O). Water is a polar solvent and can form intermolecular bonds with some alcohols.
The hydroxyl functional group in an alcohol contains one covalent bond with hydrogen and is bonded to covalently bonded to carbon. This means that it can undergo two types of reactions:
The first one in which the C—O bond can be broken by a substitution reaction or by an elimination reaction
The other one is in which the O—H bond can be broken. This makes the alcohol behave like an acid because the hydrogen ion is transferred and the oxygen is left with two lone electron pairs. The alcohol can also behave like a nucleophile.
Substitution reactions are a conversion of alcohol into a halogen alkane that involves the swapping of a halogen atom for a hydroxyl group as shown below
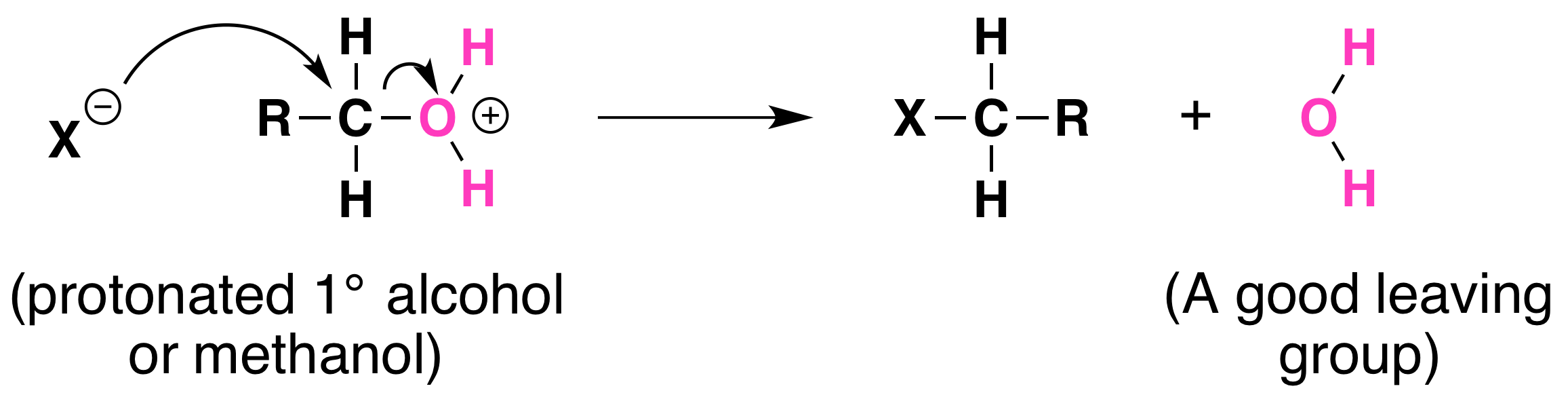 The strength of the C—O bond acts as a barrier to the substitution reaction. The only way for the substitution reaction to be successful the C—O bond to be weakened. An acidic catalyst can make this possible. A lone pair of electrons on the oxygen atom is donated to a proton in an acid-base type interaction, this leads to a very weak C—O bond and since the oxygen has developed a positive charge it is now easy to start the reaction of substitution. The reaction is normally carried out by refluxing together a mixture of alcohol, sodium bromide, and concentrated sulfuric acid.
The strength of the C—O bond acts as a barrier to the substitution reaction. The only way for the substitution reaction to be successful the C—O bond to be weakened. An acidic catalyst can make this possible. A lone pair of electrons on the oxygen atom is donated to a proton in an acid-base type interaction, this leads to a very weak C—O bond and since the oxygen has developed a positive charge it is now easy to start the reaction of substitution. The reaction is normally carried out by refluxing together a mixture of alcohol, sodium bromide, and concentrated sulfuric acid.
If an alcohol is dehydrated it forms an alkene. In dehydration, the alcohol is heated with suitable catalysts such as sulfuric acid or concentrated phosphoric acid.
The following keywords are important to memorize:
Oxidation is when an electron is removed from a molecule. In our case, it’s either a gain of an oxygen atom or a loss of two hydrogen atoms.
Reduction is the opposite where the substance gains an electron in a chemical reaction. In this case loss of oxygen or gain of two hydrogen atoms.
The substance that provides oxygen or removes 2 hydrogens from the molecule is called the oxidizing agent
The substance that removes oxygen or provides 2 hydrogen atoms is called the reducing agent.
The removal of two hydrogen atoms produces a new functional group called the aldehyde group, CHO.
In the oxidation of a primary alcohol, the reagent is acidified aqueous potassium dichromate, a mixture of potassium dichromate and dilute sulfuric acid.
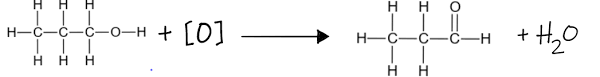
Oxidation of primary alcohols into carboxylic acids:
By changing the conditions of oxidation you can remove two hydrogens and gain one oxygen atom to produce a carboxylic acid. This reaction is represented by the following equation:
RCH2OH + 2[O] —> RCO2H + H2O
The alcohol is refluxed with acidified potassium dichromate. The acid used is dilute sulfuric acid.
In the following example you can see that ethanol is oxidized to give ethanoic acid by refluxing with acidified potassium dichromate:
CH3CH2OH + 2[O] —> CH3COOH + H2O
Oxidation of secondary alcohols into ketones:
Secondary alcohols can be oxidized to give ketones rather than aldehydes. The alcohol is refluxed with the acidified potassium dichromate. sulfuric acid is used to acidify the reagent. This reaction involves the removal of two hydrogen atoms so for example propan-2-ol is oxidised to give propanone as seen in the following equation:
CH3CHOHCH3 + [O] —> CH3COCH3 +H2O
It is impossible to oxidize a tertiary alcohol without breaking a C — C bond and so acidified potassium dichromate has no effect on tertiary alcohols.
Relative acidity of alcohols:
An acid is a proton donor. An alcohol can behave like an acid because it can donate a proton if the OH bond is broken. The weaker the O-H bond the stronger the acidity of the alcohol. The strength of the O-H bond can be changed by changing the atoms attached to the OH group. For example, the electron-releasing groups strengthen the bond and thus result in a weaker acid and vice versa.
Chapter 16: Aldehydes and ketones
Aldehydes and ketones contain one of the most important functional groups in organic chemistry, the carbonyl group which is a double bond between oxygen and carbon C=O.
In aldehydes, the carbon atom in the carbonyl group is bonded to at least one hydrogen atom, and in ketones, it is bonded to two carbon atoms.
Chapter 17: Carboxylic acids and pH
Lesson 3:
The carboxylic acids contain a carbonyl group (carbon atom double-bonded with oxygen) and an alcohol (Oxygen bonded to hydrogen).
Naming carboxylic acid:
Carboxylic has the highest priority
Start numbering of branches, doubles after CO2H end
Find the longest chain
remove ane add oic acid
For a alkene remove the e and add oic acid
hex
1 methyl
3 double bonds
4 ethyl
4-ethyl-2-methyl hex-3-enoic acid
Lesson 4:
Naming carboxylic acid
cholesterol
omega 3 and omega 6
ways to lower cholesterol
trans and cis
triglycerides and fats
pH formula
pH calculations
Notes:
Strong acids dissociate completely in water