L16-trafficking_3_notes(1)
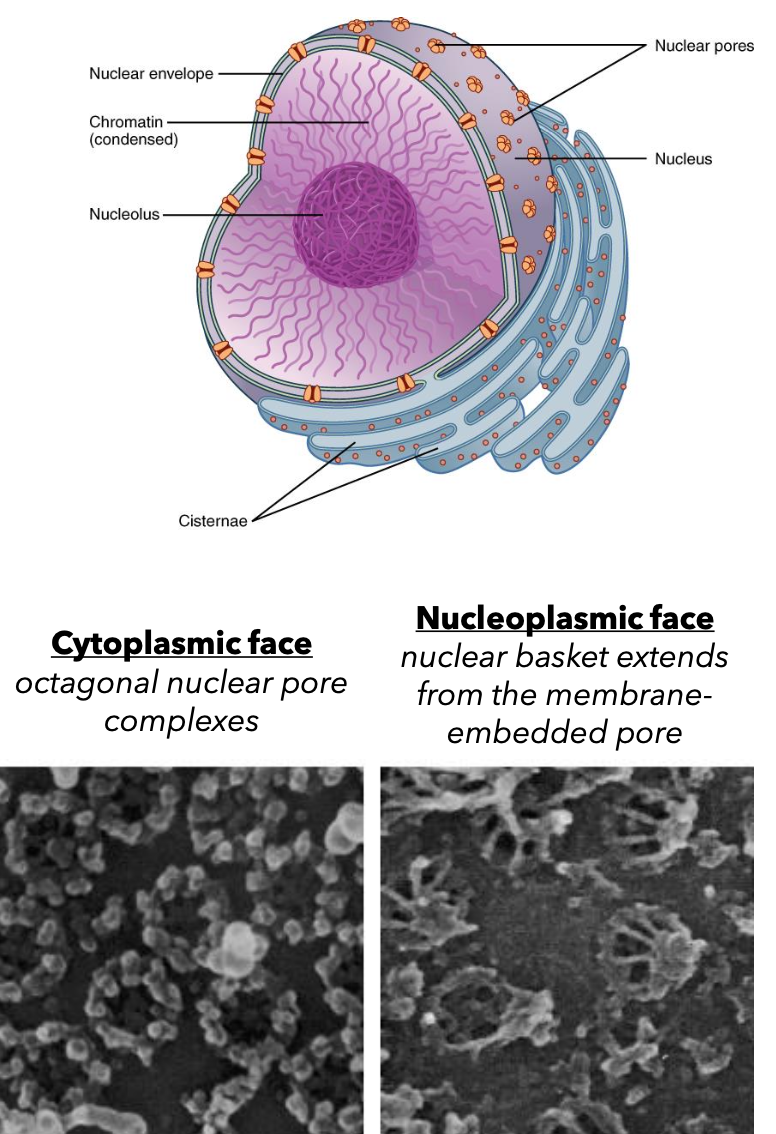
Nucleus and Its Components
Nucleus: The headquarters of the cell that contains DNA.
Nucleolus: Site of ribosomal RNA synthesis and assembly of ribosome components.
Nuclear Matrix: Composed of intermediate filaments called lamin and associated proteins, providing structural support.
Nuclear Membranes: Bound by two membranes– inner and outer membrane.
The outer membrane is connected to the endoplasmic reticulum.
Contains nuclear pore complexes for material transport in and out of the nucleus.
Chromatin: Genetic material combined with associated proteins (histones).
Nucleoplasm: Non-nucleolar regions of the nucleus.
The Nuclear Envelope Interface
Nuclear Envelope (NE): Mediates transport between nucleus and cytoplasm via nuclear pores.
Nuclear Pore Complex: Facilitates movement across the NE.
Lamina: Provides structural support to the nuclear envelope.
Continuous with the endoplasmic reticulum (ER).
The Nuclear Lamina
Nuclear Lamina: Supports the nuclear envelope; composed of lamins (intermediate filament proteins).
Integrity regulated by phosphorylation/dephosphorylation.
Human conditions caused by mutations:
Lamin A/C mutation leads to Hutchinson-Gilford Progeria syndrome.
Lamin B mutation may cause leukodystrophy (loss of myelin).
Hutchinson-Gilford Progeria Syndrome (HGPS)
HGPS: Characterized by premature aging, cardiovascular disease, kidney disease, loss of eyesight, and musculoskeletal degeneration.
Mutation creates a cryptic donor splice site;
Deletion of 150 nucleotides in mRNA.
Loss of 50 amino acids in the protein, which is improperly processed post-translationally (e.g., farnesylation target).
Dominant mutation emphasizing the significance of posttranslational modifications for protein targeting.
The Nuclear Pore Complex (NPC)
Nuclear Pores: Perforate the nuclear envelope and are composed of the nuclear pore complex (NPC).
Composed of approximately 30 proteins called nucleoporins.
Spans both membranes; size of a pore ranges from 60,000-80,000 kDa (16 times the size of a ribosome).
Dimensions: Outer diameter ~1,200 Å; Inner diameter 425 Å; Height ~800 Å.
Molecule Transport:
Small molecules and proteins (<40 kDa) can diffuse through.
Larger proteins require active transport, facilitated by soluble transport proteins interacting with cargo and pore components.
Structure of the Nuclear Pore Complex (NPC)
Membrane-Embedded Structure:
Composed of a ring structure with an aqueous pore.
Cytoplasmic and nucleoplasmic faces, with a gel-like matrix/hydrophobic sieve formed by FG nucleoporins allowing selective transport.
Nucleoporins in the NPC
Composed of 30 different proteins called nucleoporins:
Types:
Structural nucleoporins
Membrane nucleoporins
FG-nucleoporins
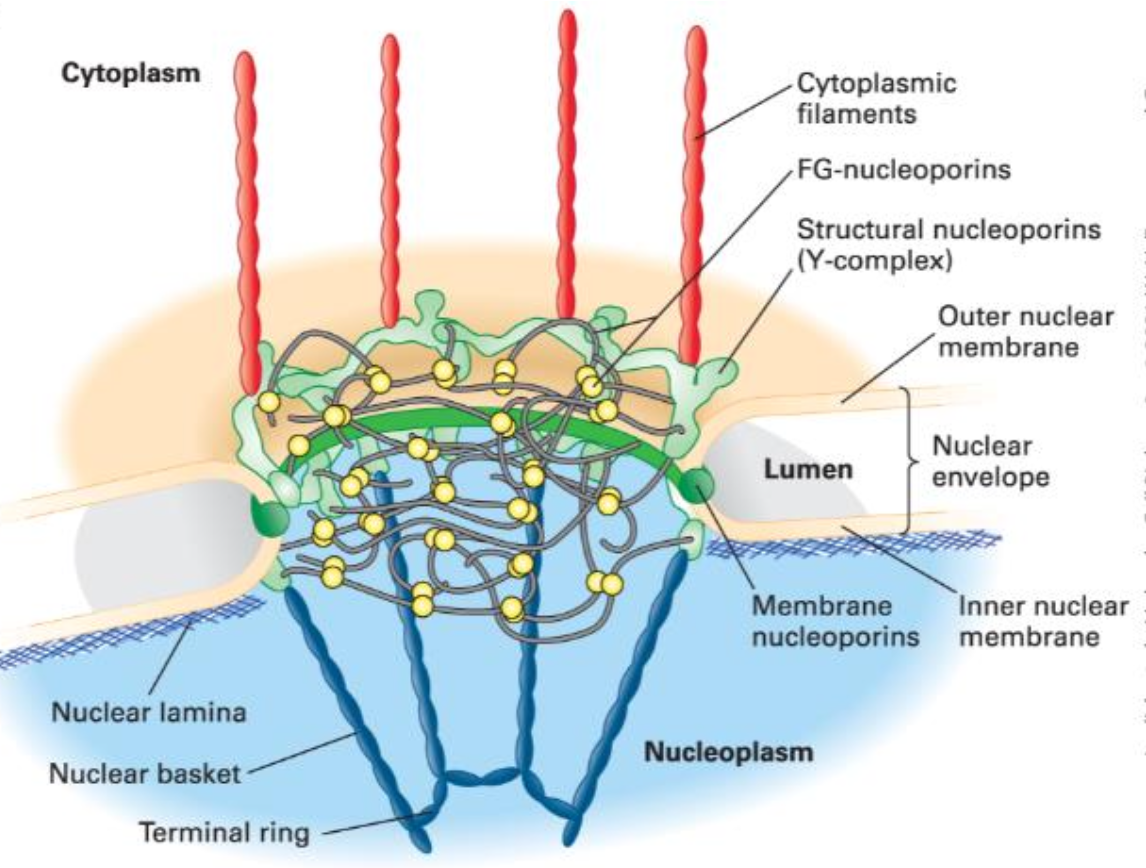
Structural Nucleoporins
Function as the scaffold for the nuclear pore, forming an octagonal ring.
The Y-complex, consisting of 7 structural nucleoporins, forms a Y-shaped structure functioning as the scaffold for the pore, about the size of a ribosome.
Membrane Nucleoporins
Connect inner and outer membranes of the nuclear envelope at the NPC via a highly curved region of membrane embedded with nucleoporins.
Associate with both structural nucleoporins and the nuclear membrane.
FG Nucleoporins Description
Contains multiple short hydrophobic sequences rich in phenylalanine (F) and glycine (G) residues.
Line the channel of the nuclear pore complex.
Found in nuclear basket and cytoplasmic filaments.
Forms a highly disordered structure that functions as a gel-like matrix/hydrophobic sieve, allowing small molecule diffusion while blocking unchaperoned transport of larger proteins (>40 kDa).
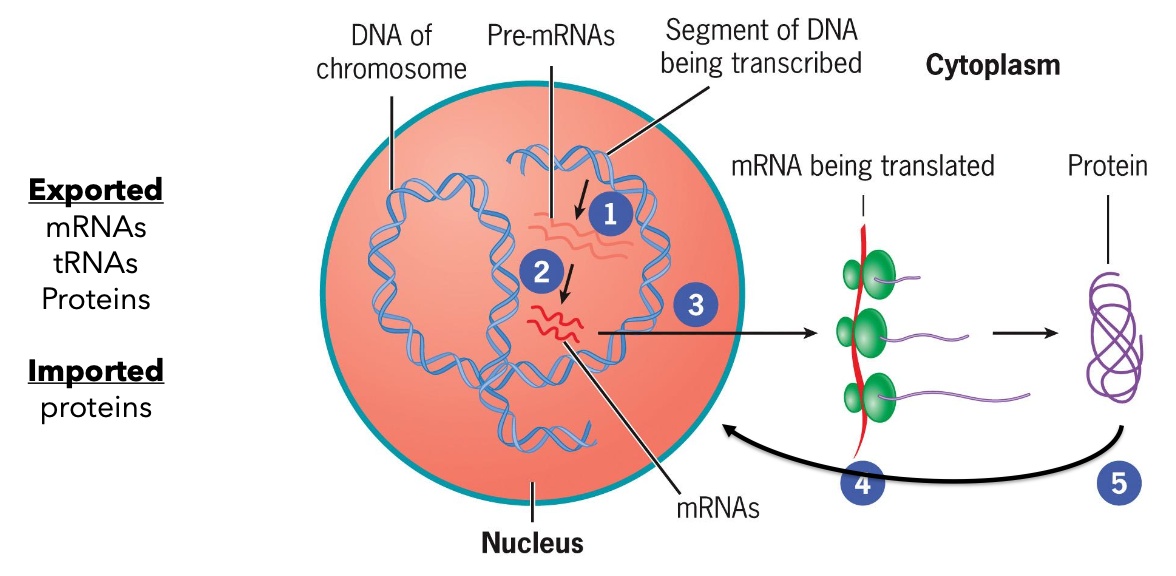
Page 17: Molecular Trafficking
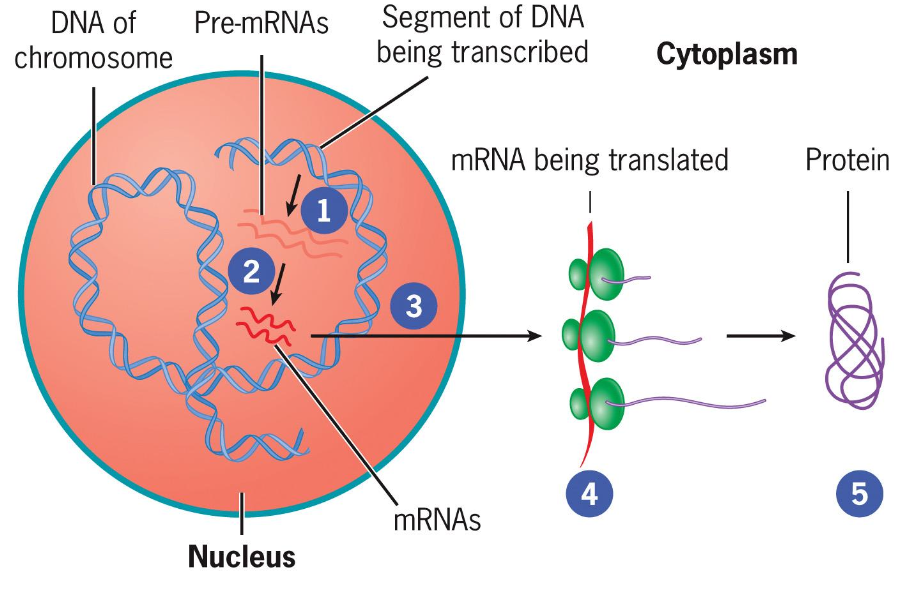
Pathway of mRNA Transport:
DNA (Pre-mRNAs) transcribed in the nucleus.
mRNA is translated in the cytoplasm.
Exported items include mRNAs, tRNAs, and proteins export/import activities.
Review of Transcription
Shows the interaction of RNA polymerase and the transcription bubble, leading to RNA strand synthesis.
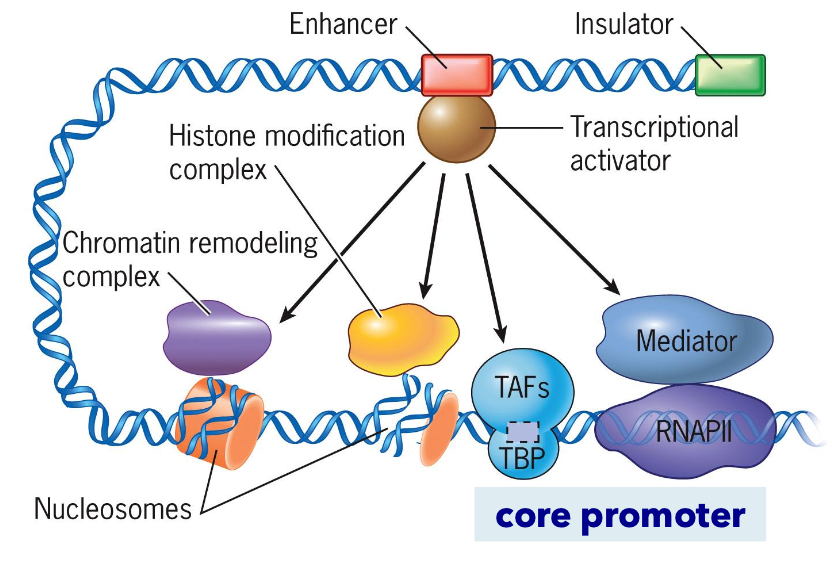
Transcription Initiation
Involves RNA polymerase II promoters and general transcription factors:
TBP: TATA binding protein.
TAF: TBP-associated factors.
Additional components influencing transcription include:
Enhancers, insulators, histone modifications, transcription complexes, and chromatin remodeling complexes.
Spatial Organization of Genome
Chromosome territories show activity levels:
Chromosome 19: More active with protein-coding genes.
Chromosome 18: Less active with fewer coding genes.
Heterochromatin (inactive) at the periphery; euchromatin (active) at the interior.
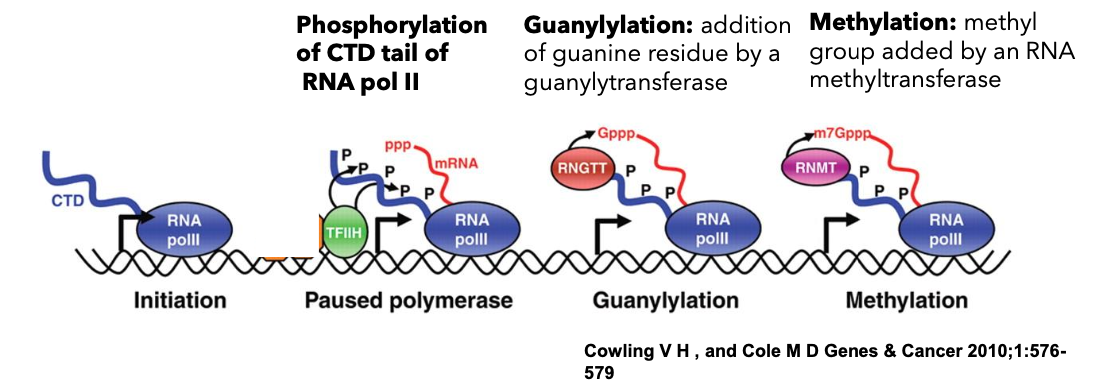
mRNA Processing Steps
Pre-mRNAs become mature mRNAs through:
5’ methylguanosine cap addition.
Prevents the 5’ end of mRNA from degradation.
Aids in transport of mRNA out of the nucleus and initiation of mRNA translation.
Formation involves:
Phosphorylation of CTD tail of RNA pol II.
Guanylylation by guanylytransferase.
Methylation by RNA methyltransferases.
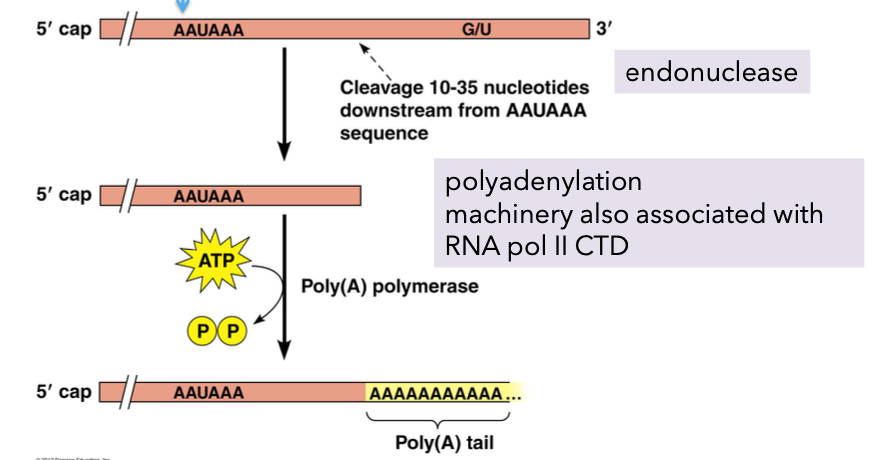
Poly A tail formation.
Role in protection from rapid degradation of mRNAs lacking poly A tails.
Machinery associated with RNA pol II assists in end processing:
CTD endonuclease assembly site
Splicing.
Structure of Mature Eukaryotic mRNA
Key features:
Continuous sequence encodes specific polypeptides.
Noncoding sequences: 5’ UTR, 3’ UTR.
Modifications at ends: 5’ methyl guanosine cap, 3’ poly A tail.
mRNA Exit from the Nucleus to be translated
Components:
Nuclear envelope (inner and outer membranes).
Nuclear pore complex.
Nucleolus as rRNA synthesis site.
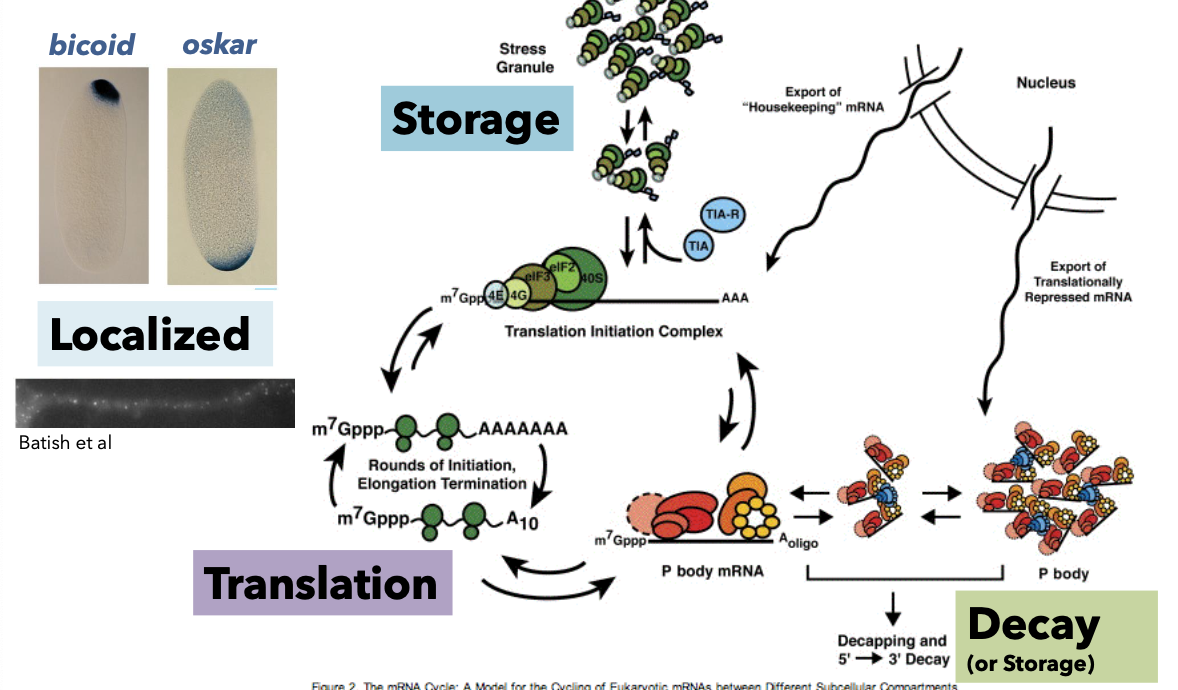
Fate of mRNA after Processing
mRNA Trafficking Summary
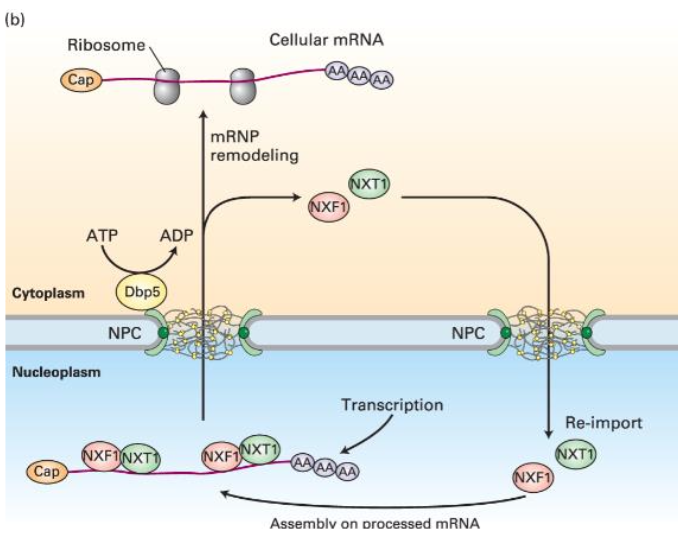
mRNA Export Process
Proteins associate with mRNA, forming mRNP (mRNA-protein complex) co-transcriptionally.
Export facilitated by the mRNP Exporter with multiple components including NXF1 (large subunit) and NXT1 (small subunit).
Ran-Independent Mechanism for mRNA Export
Involves:
Binding of NXF1/NXT1 to processed mRNPs, acting as quality control.
Interaction with FG-repeats in NPC for transport.
RNA helicase promotes RNP dissociation aiding transport to the cytoplasm.
How do proteins get into the nucleus?
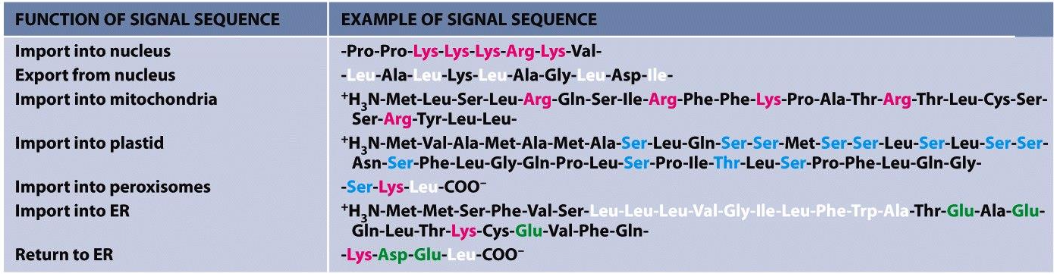
Signal sequences in amino acid sequences or attached signals (e.g., carbohydrates) dictate import.
Receptors bind to signals, facilitating targeting.
Signal sequences in amino acid sequences or attached signals (e.g., carbohydrates) dictate import.
Receptors bind to signals, facilitating targeting
Signal sequences in amino acid sequences or attached signals (e.g., carbohydrates) dictate import.
Receptors bind to signals, facilitating targeting.
Nuclear Import Process via NPC
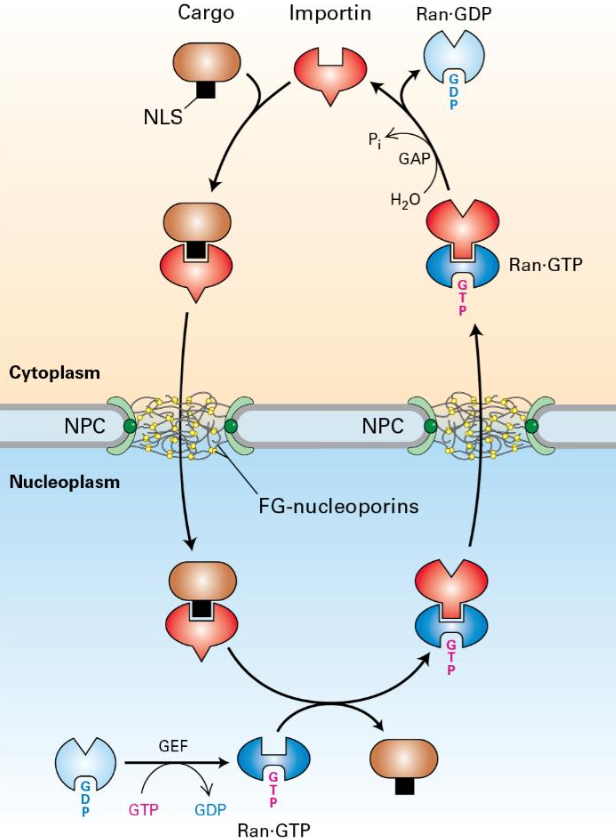
Import requires nuclear localization signals (NLS) that consist of 5 positively charged amino acids (e.g. Lys, Arg).
Importin receptors (nuclear transport receptors) bind to NLS and facilitate transport through the nuclear pore via interaction with FG-repeats.
Steps of Protein Import into the Nucleus
Importin binds NLS cargo in cytoplasm.
Complex moves through NPC via interaction between importin and FG repeats.
Cargo dissociates upon interaction with Ran:GTP in the nucleus.
Importin-Ran:GTP returns to the cytoplasm via NPC.
Ran:GDP diffuses back to nucleus.
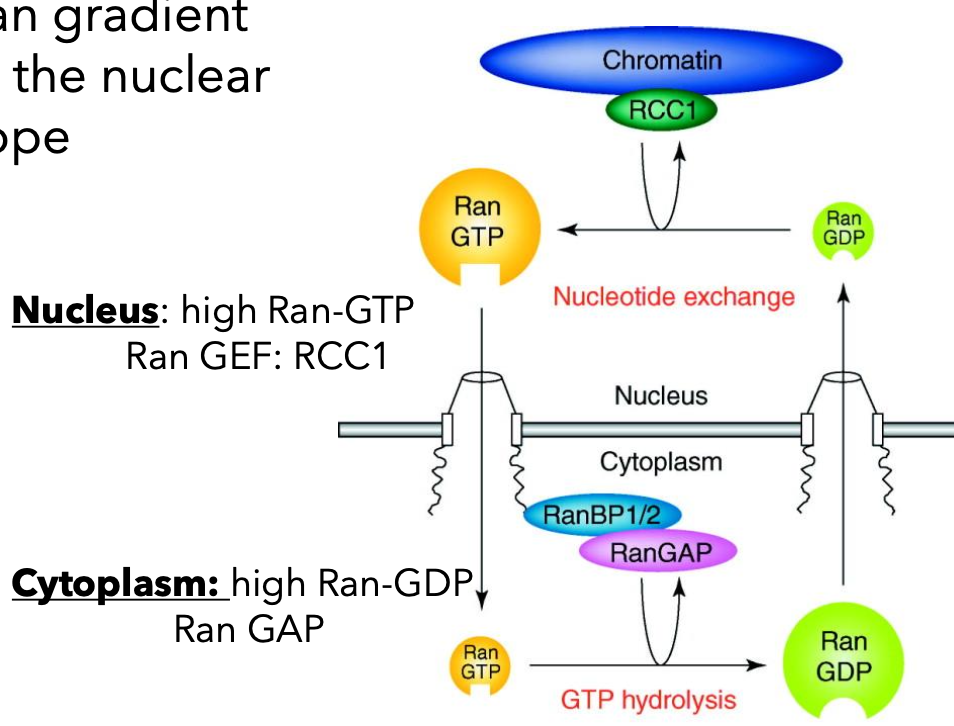
Ran Gradient
High Ran-GTP concentration in the nucleus regulated by Ran GEF (RCC1).
Provides energy for unidirectional export through the nuclear pore by hydrolyzing GTP.
How are proteins transported out of the nucleus?
Proteins possess nuclear export signal sequences, often rich in leucine (e.g. PKI, Rev).
Nuclear Export Signal (NES), a leucine rich sequence, recognized by exportins (nuclear transport receptor) that facilitate movement across the nuclear pore by interacting with FG-repeats.
Steps of Protein Export
Exportin 1 forms a complex with Ran:GTP in the nucleus.
NES binding to cargo is enabled.
Cargo diffuses into the cytoplasm.
Ran:GAP stimulates GTP hydrolysis.
Low affinity causes dissociation in the cytoplasm and exportin returns to nucleus.
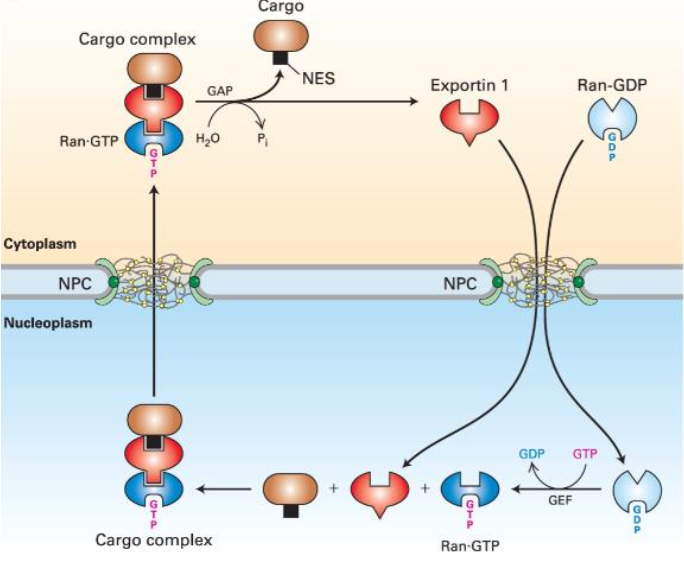
Cycles of Nuclear Import and Export
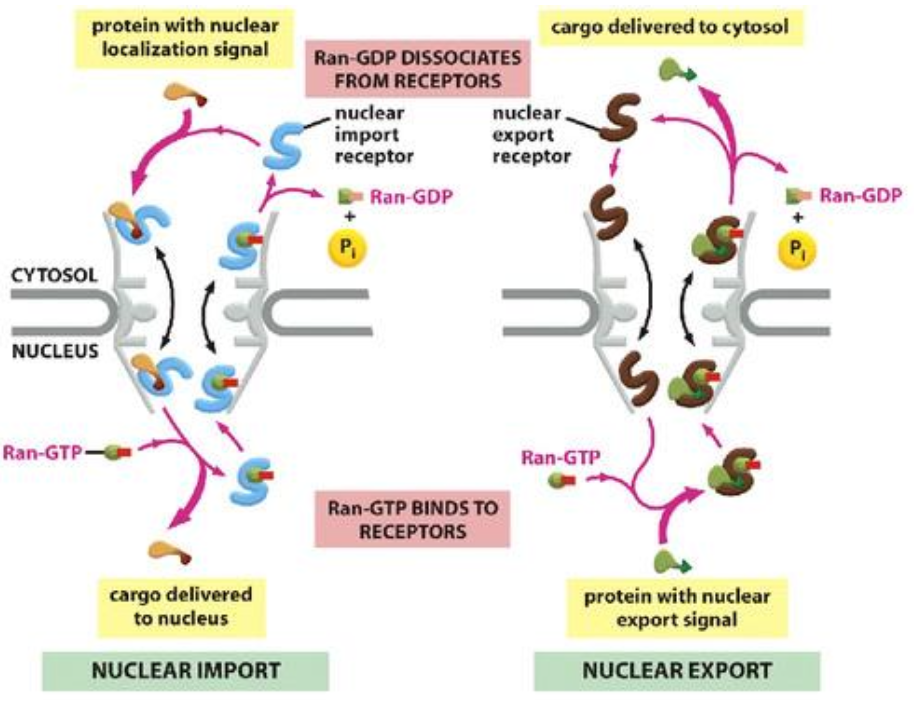
Continual cycling process facilitated by the presence of nuclear localization signals for import and nuclear export signals for export.