Chapter 3 Chemistry Review
3.2 Video Notes
Model: A familiar idea used to explain unfamiliar acts observed in nature
Theory: An explanation of observed facts & phenomena
-explains all known facts
-enables scientists to make correct predictions
Democritus proposed the existence of an atom
-word comes from the Greek word “atonnis” which means “not to cut” or “indivisible”
Aristotle rejected the idea of the atom
-said matter could be cut continually
Dalton’s theory proposed that atoms:
-are building blocks of matter & are indivisible
-of the same element are identical & different for different elements
-unite in small, whole-number ratios to form compounds
J.J Thomson
-credited with the discovery of electrons: a blow to Daltons indivisible atom
-proposed the “plum pudding" model of the atom: negatively charged electrons embedded in a ball of positive charge
Rutherfords Gold-foil Experiment:
-aimed alpha particles at gold foil & most particles passed through
-a few particles deflected & some particles bounced back
-concluded that most of the atom is empty space & has a dense positively charged core
3.3 Atomic Structure Notes
Rutherford referred to the area that is dense positively charged center is where all the mass is concentrated in an atom is known as the nucleus
-composed of protons & neutrons (Chadwick helped Rutherford with the idea of a neutron)
-Electrons are distributed around the nucleus (taking up most of the volume)
-known as the nuclear model

Protons have a positive charge & relative mass of 1.007276 amu
Neutrons have no charge & a relative mass of 1.008665 amu
Electrons have a negative charge & a relative mass of 0.0005846 amu
-the space electrons move in accounts for most of the atomic volume
-they’re the parts that “intermingle” when atoms combine to form molecules
The number of electrons an atom has affects the way it can interact with other
atoms- Atoms of different elements with different numbers of electrons = different chemical behaviors
The atomic number of an element comes from the number of protons in the nucleus
An atom’s mass number is found by taking the sum of the number of protons & neutrons in a nucleus
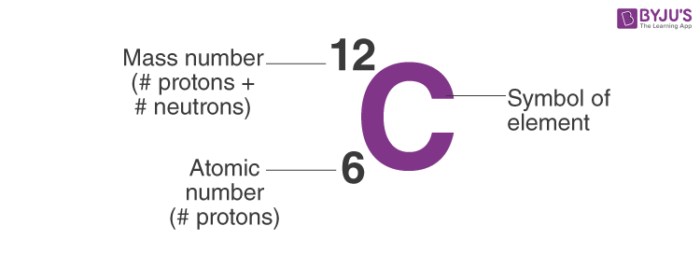
Atoms are composed of identical protons, neutrons, & electrons
Elements are different because they contain different numbers of protons
# protons (in a neutral atom) = # electrons
Atoms of a given element with a different number of neutrons & the same amount of protons are called isotopes
-different masses

3.4 Introduction to the Periodic Table Notes
The periodic table is an arrangement of elements in which elements are separated into groups based on a set of repeating properties
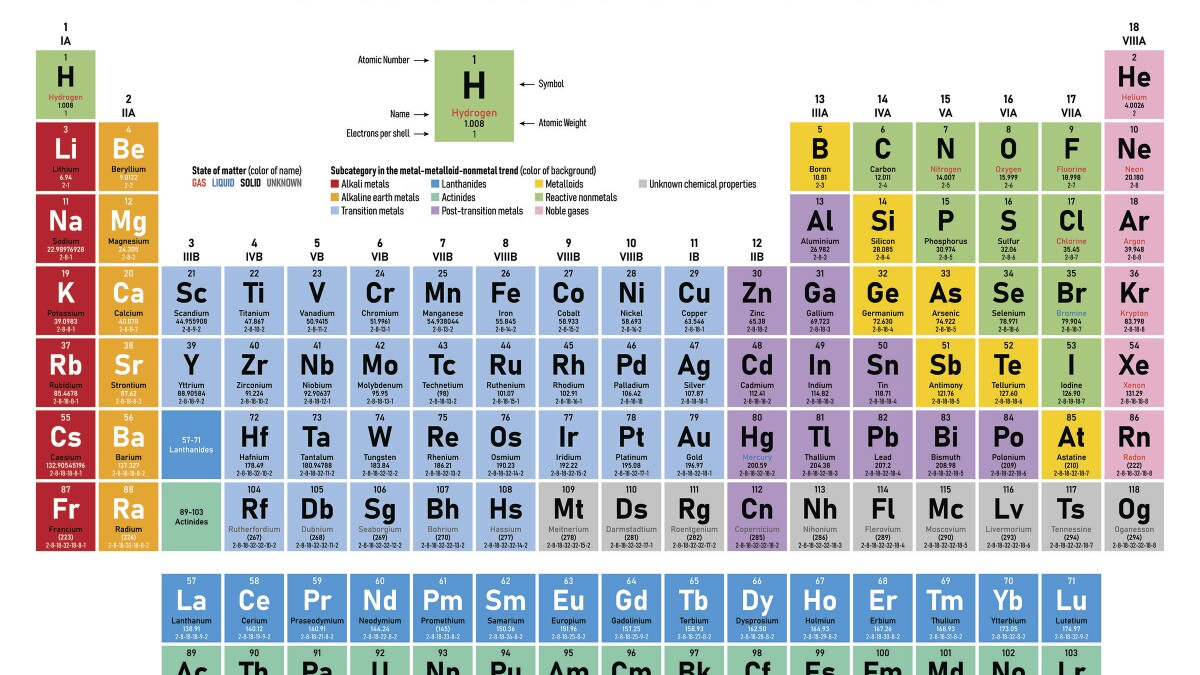
Elements are listed on the periodic table in order of increasing atomic number
The horizontal rows of the periodic table are called periods & the vertical columns are called groups or families
-the elements in any group of the periodic table have similar physical & chemical properties
Alkali Metals: silvery appearance, soft, very reactive, not found as free elements in
natureAlkaline Earth Metals: harder, stronger, denser, & less reactive compared to alkali metals
Transition Metals: good conductors of electricity, high luster, less reactive, & some free elements in nature
Halogens: most reactive nonmetals, react w/ metals to form salts, & they exist as diatomic molecules
Noble Gases: have filled energy levels & are not interested in reacting, don’t
readily combine with other elements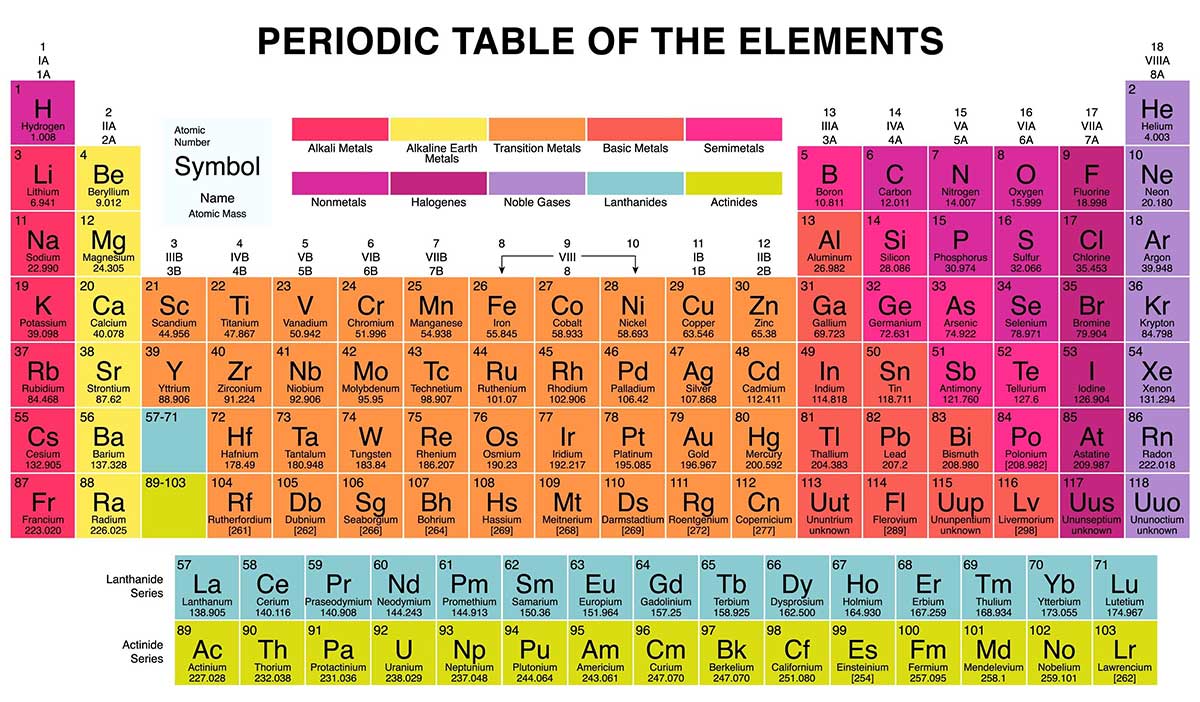
Most of the elements on the period table are metals
-Efficient conductors of heat & electricity
-Malleable (can be hammered into thin sheets)
-Ductile (can be pulled into wires)
-Lustrous appearance (shiny)
-Mostly solidsA relatively small group on the periodic table are nonmetals
-Lack the properties that characterize metals, they’re poor conductors of heat & electricity
- Show more variation in their properties than metals
- Many are gaseous, only a few are solids, & Bromine is a liquid.Metalloids lie close to the “stair-step” on the periodic table
- Often shows a mixture of metallic & nonmetallic properties
- Sometimes called semimetals
- Have characteristics of both metals & nonmetals (Semiconductors)
🡪B, Si, Ge, As, Sb, Te, & Po.
(Depending on the resource)
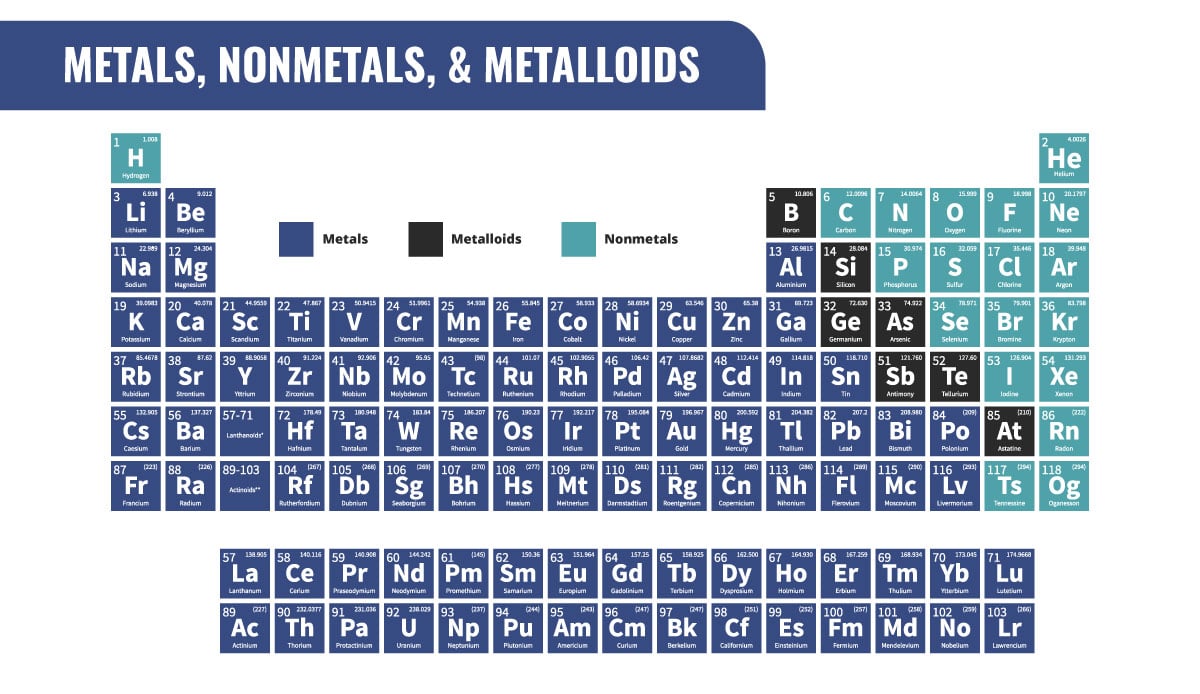
Atomic Radius: the total distance from an atom's nucleus to the outermost
orbital of an electron & increases to the LEFT & DOWN
Ionization Energy & Electronegativity: the energy required to remove an electron from a neutral atom Increases UP & to the RIGHT

Elements are not typically found in nature in their pure form.
Diatomic molecule: A molecule composed of two atoms
Diatomic element: An element that does not exist by itself & will combine with atoms of the SAME element to be stable
-These elements are diatomic ONLY when they are alone, NOT when chemically
bonded to another atom:max_bytes(150000):strip_icc()/what-are-the-seven-diatomic-elements-606623-v3-5b562dab46e0fb0037fee8c7.png)
Only 2 elements are liquids in their elemental forms at 25 degrees Celsius
-Bromine: heavy, reddish-brown liquid is the only non-metallic
element that is liquid at room temperature-Mercury: heavy, silvery liquid, only metallic element that is liquid at room
temperature
3.5 Ions/Valence Electrons Notes
An atom that has gained or lost e- & thus acquired an electrical charge is called an
ion-Ions can be anions or cations
Octet Rule: An atom will gain, lose, or share electrons to have 8e- in its outer shell (valence shell)
-Having 8 valence e- makes most atoms stable-Exceptions–H & He are stable with 2, other exceptions are in period 2
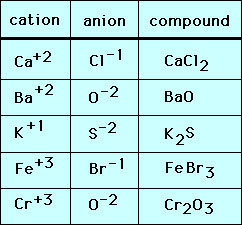
Cation: positive ions from one or more electrons are lost from a neutral atom
-named by their “parent atom” with a +
Anions: negative ions from a neutral atom gaining one or more electrons
-named by the root of their parent atom, adding the suffix ~ide, & a -
Ions are NEVER formed by changing the number of protons in the atom’s nucleus
Isolated atoms don’t form ions on their own, usually, ions form when metallic elements combine with nonmetallic elements
Metal atoms tend to lose one or more electrons becoming cations which are, in turn, gained by the atoms of the nonmetal atoms forming anions
The Group # (column #) is the # of valence e- that all atoms in that group have
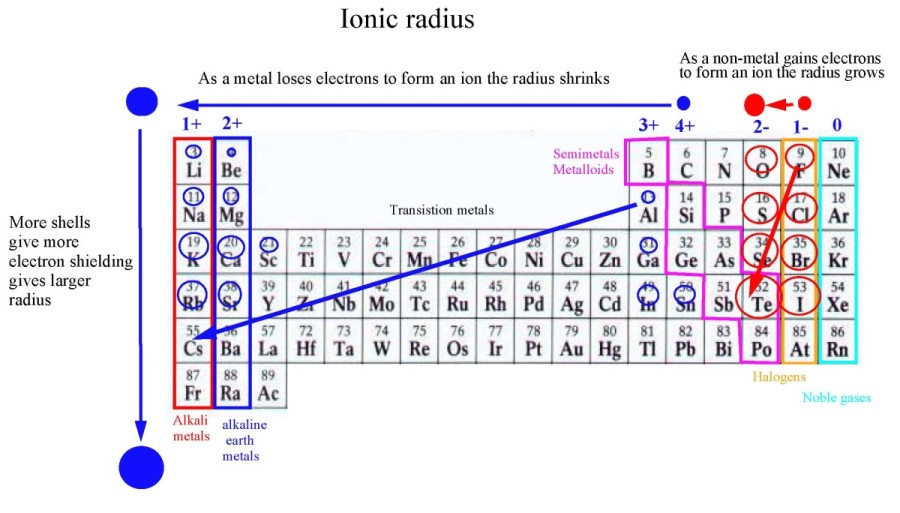
Valence electrons occupy the highest energy level of an atom
-involved when atoms attach to one another
Chemical bonds link atoms together, making them act as units
-bond because they are more stable together than alone
Electrons play a crucial role in forming bonds
-Only the valence(outer shell) e- are involved, Inner core electrons
e- do not form bonds
• Bonds are formed when atoms transfer or share their valence electrons
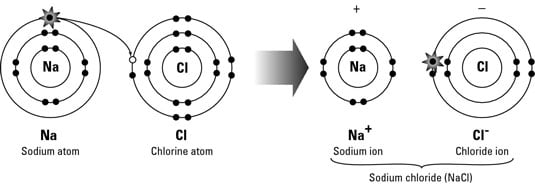
Ionic Compound: results when a metal reacts with a nonmetal to form cations
& anions (requires a transfer of electrons, gaining or losing)The formula for ionic compounds = Both cations & anions must be present & net charge of the compound must be zero.
-Electrically Neutral Overall
Formulas for ionic compounds give the ratio of ions, not the total # of ions
– In the ratio of cations:anions the charges will add & leave the compound with a 0 net charge