4.5: Application of reproduction + genetics
Genome projects
Human genome
The sequencing of the human genome was proposed in 1985, and the project began in 1990.
It was planned to take 15 years, but rapid DNA sequencing and computing advancements allowed a draft to be published in 2000, which was completed in 2003.
Analysis of it’s data still continues.
It was done to improve understandings of genetic disorder diagnosis and treatment.
It aimed to:
Identify all genes in the human genome, and the chromosome they originate on.
Determine the sequence of the 3 billion base pairs in human DNA.
Store this information in databases.
Improve data analysis tools.
Transfer related technologies to the private sector, to develop medical innovation.
Address social, legal and ethical issues that may arise.
It found:
Humans have around 20,500 genes, which is fewer than expected.
There are more repeated segments of DNA than previously expected.
Fewer than 7% of the families of proteins were vertebra specific.
Sanger sequencing
The project used Sanger sequencing, AKA the chain termination method.
He won his second nobel prize for this discovery.
Good video explanation:
Sangar designed this method via the removal of both the 3’OH and the 2'OH, as this would cause the DNA polymerase to no longer be able to bind the next nucleotide.
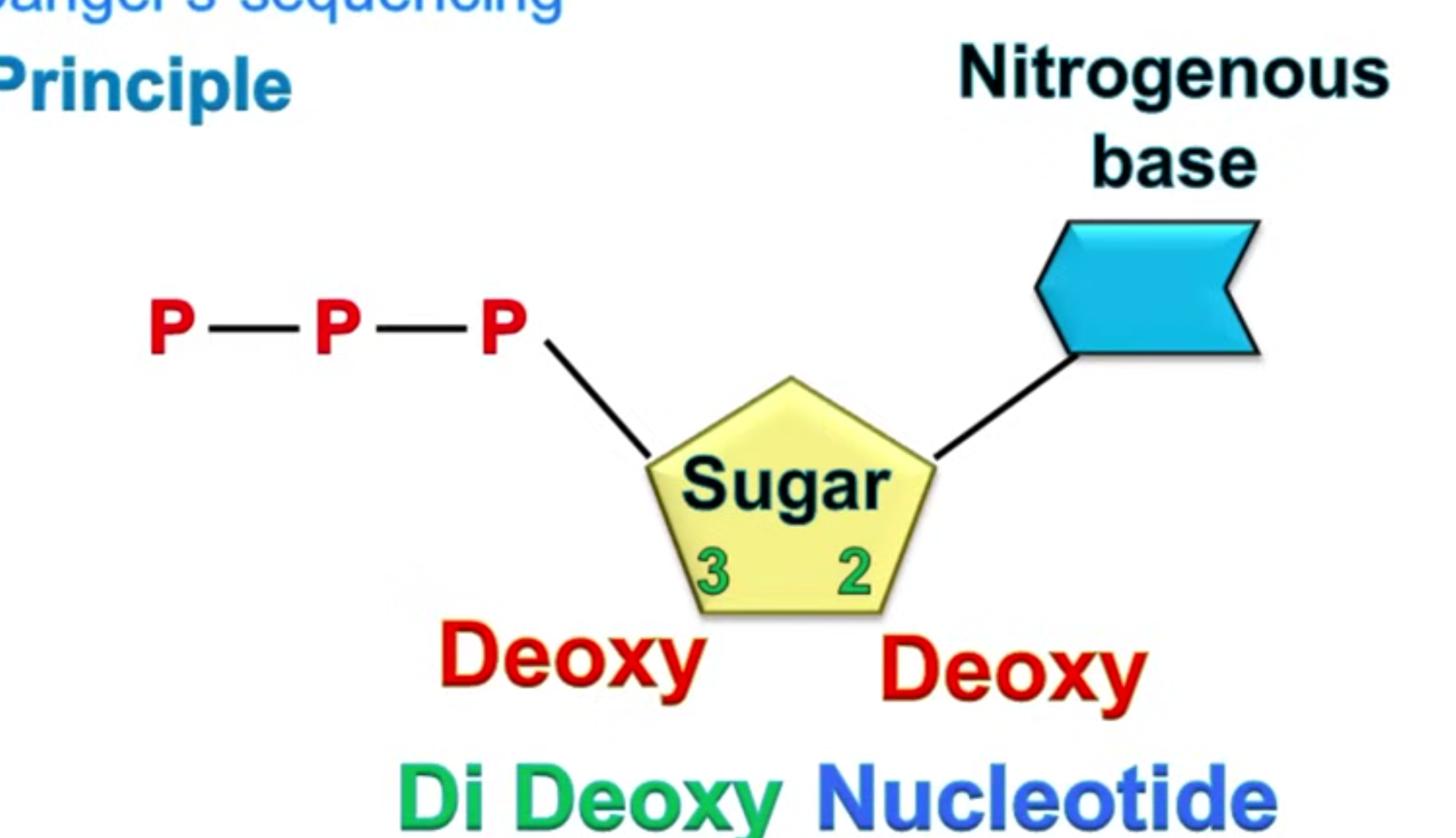
It involves:
DNA is broken up into single-stranded fragments of different lengths, around 800 bases long, and placed in separate tubes.
Complementary strands are synthesised, but are incomplete due to the four nucleotide triphosphates (NTP - the 4 bases, G, C, T and A) being incomplete.
In each tube, one NTP will be made into a dideoxynucleotide marker, comprising an antigen, radioactive isotope or fluorescent marker. This will be different for each of the 4 nucleotides.
DNA polymerase codes the chain as normal until it reaches this marker, and is then unable to bind to the next nucleotide and the chain is terminated.
From each original DNA fragment, a large number of complementary DNA strands of increasing length were produced.
They were separated by gel electrophoresis according to their size, and the marked (terminal) nucleotide was identified.
As all the terminal nucleotides were known, in different size fragments, these were then able to be compared in order to determine the base sequence of the original DNA fragment.
Using this method, it took a year to sequence a million base pairs.
There are many new techniques, known as Next Generation Sequencing (NGS) and can sequence an entire genome within a few hours.
An example is passing DNA through micropores in protein molecules.
100K Genome Project
This project was launched in 2012, following the success of the Human Genome project.
NGS techniques were used to sequence 10,000 genomes from NHS patients, or relatives, with a rare disease or cancer.
It is run by Genomics England, under the Department of Health, and aims to:
Create a ethical and transparent programme based on consent.
Set up a genomics programme in the NHS, which will benefit patients directly.
Enable medical and scientific discoveries.
Develop a UK genomics industry.
By combining sequenced data with medical records, the causes of diseases are expected to be recognised and understood, and their diagnosis and treatment improved.
Ethical and moral concerns
A vast quantity of data has been produced by these projects, and how, where and issues with this data are debatable:
Ownership of genetic information - If once decoded, this information is owned by an individual there must be safeguards to prevent it’s misuse.
Insurance should not have access to information which may affect it’s pricing, such as a heart disease gene.
DNA ancestry should not be used in a discriminatory way.
No company should profit from using this information without consent.
If mutations are identified which are correlated with certain health issues are identified, do close relatives have a right to this information?
Genetic screenings can be used alongside genetic counsellors to allow people to understand the potential risks to themselves or potential children.
Without this information, the counsellor bases their advice on family members with the condition, frequency of the gene within the general population and how closely related the family members are.
Embryos made via in vitro fertilisation (IVF) can be screened for alleles which cause conditions such as cystic fibrosis.
This allows people to implant only healthy embryos. Legal frameworks exist for the carrier ‘spare‘ embryos to be used in research.
Embryo screening could also screen for desirable characteristics, such as appearance and intelligence. However, these genes are a result of multiple alleles and epigenetic factors, and even the specific alleles are unknown.
This could suggest these characteristics cannot be selected for via allele selection or the environment.
If these techniques are developed, however, there must be discussions over who can decide whether alleles can be selected for.
Parent’s can have their children screened for predispositions to future diseases, but when should a child be told this information?
Additionally, do the parents have a right to this information, and if so does this mean a child’s DNA is the property of a parent.
DNA must be stored and secured against hacking, as it is often stored on computers.
Non-human animal DNA sequencing
Genomes have been sequenced from organisms from all domains and kingdoms of life, and a significant number of phyla.
Species coded are chosen for their economic or cultural significance.
Examples include the first organism - MS2, and the first eukaryote - baker’s yeast.
The analysis of closely related organisms allows inferences of evolutionary relationships to be made.
These provide true phylogenetic classification, and are compared with schemes based on phylotypic characteristics.
Primate research is of particular interest as this links to the understanding of human origins.
These comparisons also indicate to conservationists which species need particular protection.
Malaria
Malaria is common in tropical and subtropical regions around the equator, including sub-Saharan Africa, Asia and Latin America.
In 2-13, WHO reported 198 million cases with over half a million deaths, 90% of which were in Africa.
To attack the illness, both the vector and parasite have been researched.
Vector - Anopheles gambiae
Insecticides are used to kill mosquitoes indoors, but resistance is a major issue as once resistant to one insecticide, they are resistant to all insecticides in that particular class.
Pyrethroid resistance is a major issue, as it is the only one recommended alongside mosquito nets people sleep under to protect them from mosquitoes.
DNA sequencing of mosquitos occurred in 2002, which was done to develop chemicals which make them susceptible to insecticides again.
In 2015, a genetically modified mosquito was produced which gave them a gene allowing them to synthesise an antibody against the parasite, Plasmodium.
This was done using a gene editing technology known as CRISPR-Cas9 which allows genes to be ‘written into‘ a genome.
Mosquito eggs had this gene ‘added‘.
This would cause a mosquito to kill the parasite once it contracted it from feeding from an infected person.
This would prevent the spread of malaria.
In the lab, 99.5% of the offspring carried the gene, but it was not released into the wild, but provided a model for future progress in malaria control.
Parasite - Plasmodium falciparum
Drugs to kill this parasite originate back to the early 17th century, when extracts from Cinchona bark were used.
They contained quinine, which disrupts the parasite's digestion of haemoglobin in red blood cells.
A toxic derivative of haemoglobin accumulates and kills Plasmodium.
The parasite has become resistant to quinine and other drugs.
These can be developed by a single or multiple mutations.
Over time, this becomes developed in populations and can be very stable, persisting even once the drug is out of use.
Example include:
The drug Chloroquine, which disrupts the digestion of haemoglobin in the parasite’s food vacuole.
Resistant parasites expel Chloroquine from its food vacuole 50x faster than normal, so the drug cannot have its effect, making it resistant.
Atovaquone kills Plasmodium by acting on it’s ETC.
Resistance forms rapidly via a single point mutation on the gene for cytochrome b.
Artemisinin, from the sweet wormwood Artemisia is used in combination with other drugs, by acting on Plasmodium in the red blood cells, although this is not fully understood.
Resistance has been detected to this.
The genome of this parasite was sequenced in 2002.
It is hoped this will allow for the development of more effective drugs.
Genetic fingerprinting
Around 99.9% of the human genome is identical, and it is only 0.1% which differentiates genetic profiles.
Genetic fingerprints represent the non-coding portions of DNA (introns), which is therefore different from DNA sequences.
Two techniques are used during the process:
PCR to make large numbers of copies of DNA fragments.
Gel electrophoresis, to separate DNA fragments based on size.
DNA used
Introns have sequences of nucleotides in which up to 13 bases repeat up to several hundred times.
These are called short tandem repeats, or STRs.
The number of repeats within an STR is different in different individuals, which is what makes genetic fingerprints unique.
The number of repeats is inherited and can be used for identifying people and for tracing family relationships.
Polymerase chain reaction (PCR)
This is a semi-conservative DNA replication of DNA in a test tube, which makes millions of copies and works rapidly.
This makes PCR highly useful with small or degraded samples.
The DNA sample is dissolved in a buffer and mixed with:
Taq polymerase, which is from the Thermus aquaticus bacterium, which lives in hot springs.
This is due to it’s high optimum temperature of 80 degrees, and ability to remain active for 9 minutes in 97.5 degree conditions.
Nucleotides containing the 4 bases.
Single short stranded pieces of DNA between 6-25 bases long known as primers.
They are complementary to the start of the DNA strand and bind to it, signalling taq polymerase action.
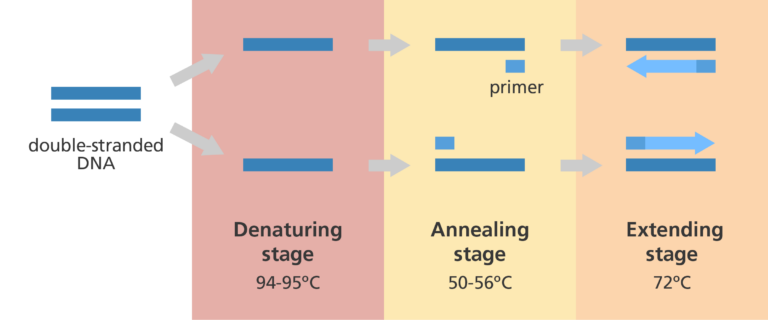
The technique requires rapid temperature change, and therefore happens in a thermocycler device.
It occurs in 4 stages:
The original DNA is heated to 95 degrees, separating it into two strands.
The solution is then cooled to 55 degrees, which is cool enough for primers to bind to the complementary base sequences on each of the single DNA strands.
The solution is heated to 70 degrees, and taq polymerase catalyses the synthesis of a complementary strand by adding complementary nucleotides and catalysing the formation of phosphodiester bonds in the sugar-phosphate backbone.
This is the elongation or extension phase.
For each original fragment of double stranded DNA, two identical double strands are produced.
The sequence is repeated many times.
Limitations
There is another method, where copies of genes are made by inserting them into replicating microorganisms, which is still sometimes used due to these limitations:
Contamination - Any DNA that accidently enters the system can be amplified.
This can be airborne, from the experimenter or contaminated reagents, but is most often from contaminated apparatus from previous PCRs.
Error rate - All polymerases sometimes accidentally insert a nucleotide containing the wrong base, which is normally corrected during proofreading, but taq polymerase cannot do this.
It makes an error 1/9000 nucleotides, but after 30 cycles this can raise to 1/300.
This is due to errors being multiplied by previous errors
DNA fragment size - PCR is most efficient for making DNA 1000-3000 base pairs long due to taq polymerase being unable to correct errors.
A base pair length of 40,000 can be generated if a lower temperature, higher pH and proofreading polymerase is also added.
But many genes, including human genes, are longer than this.
Sensitivity to inhibitors - PCR is very sensitive to molecular inhibitors, including:
Phenolics, especially in plant material.
Humic acids in archaeological remains.
Haem breakdown products, which bind with Mg22 needed for DNA polymerase to function.
The traditional blue dye used in denim.
Limits on amplification - After about 20 cycles, the exponential DNA molecule increase slows down, becoming linear and then plateauing due to:
Reagent concentrations becoming limiting.
Enzyme denaturing after repeated heating.
DNA in high concentrations causes the single-stranded molecules to base pair with each other over the primers.
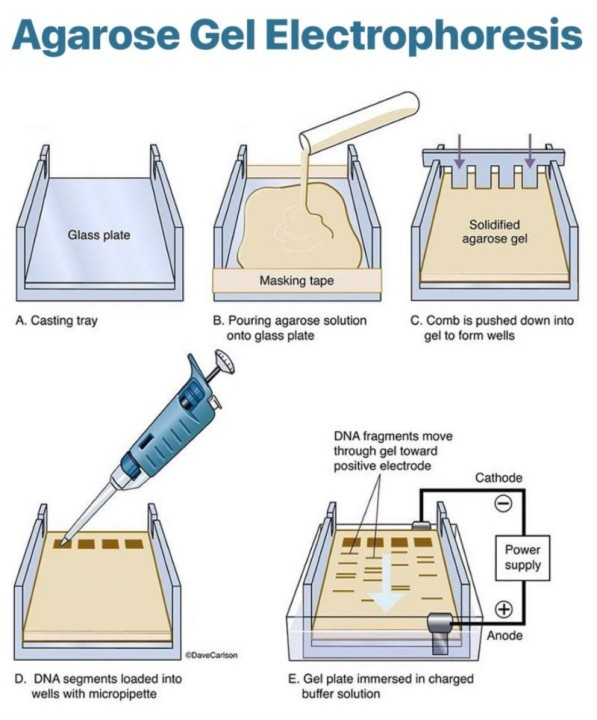
Gel electrophoresis
DNA is extracted and cut into thousands of fragments of varying lengths via restriction endonucleases.
They are then separated by length via gel electrophoresis, on an agarose gel.
This gel is a polysaccharide extracted from seaweed and makes a gel with pores, through which small molecules can move.
It occurs in 3 steps:
DNA samples are loaded into wells at one end of the gel.
A voltage is applied across the gel.
Phosphate groups of the DNA backbone have a negative charge, so they are attracted to the anode.
Smaller fragments move more easily through pores, and so they migrate through the gel faster than the larger fragments.
If fragments of known lengths are separated on the same gel at the same time, making a DNA ladder, the lengths of the fragments under test can be estimated.
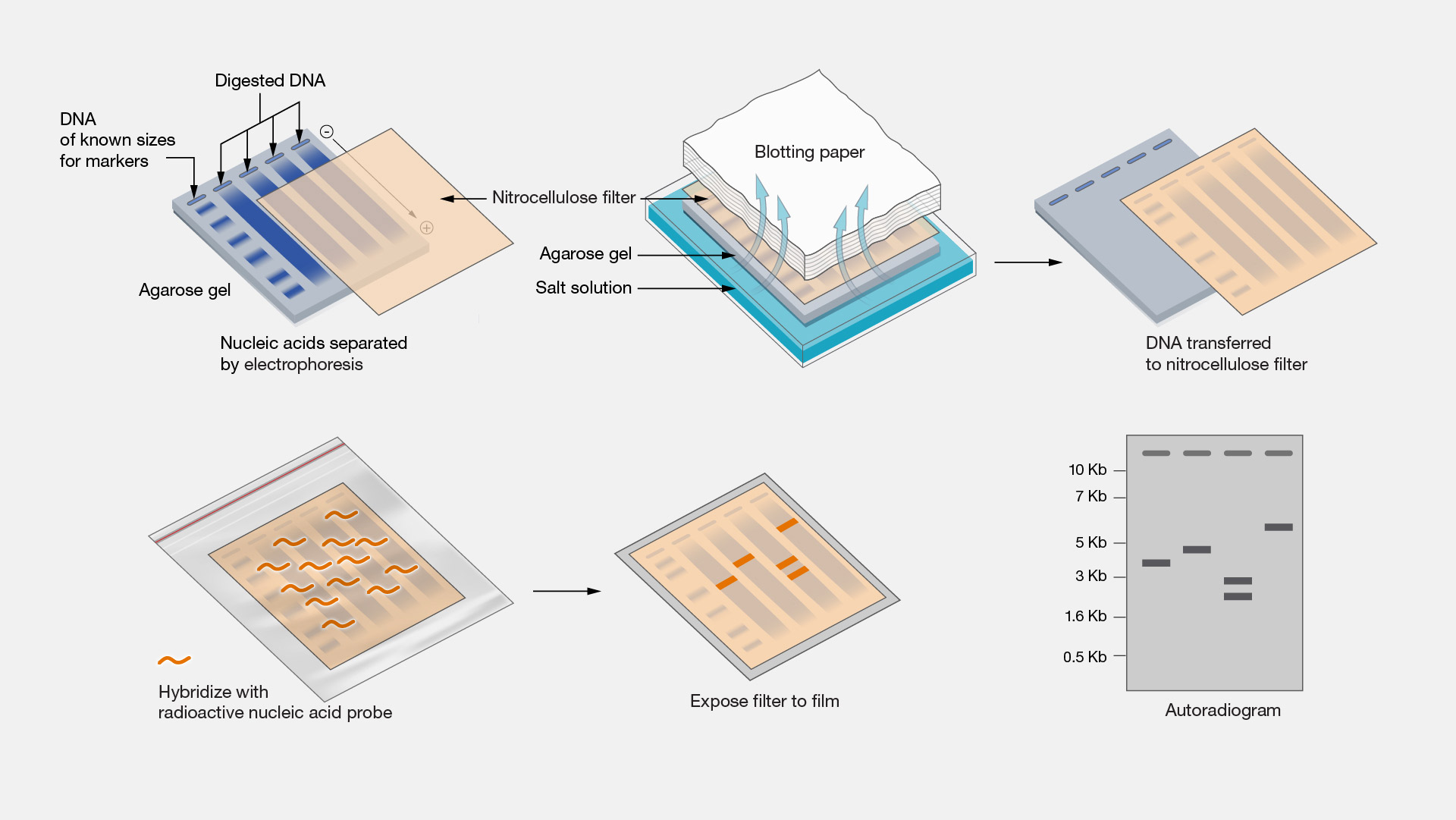
Next, in order to make a visible fingerprint:
The electrophoresis trough is covered with a nylon membrane, which touches the gel and picks up the DNA fragments, in a process known as Southern blotting.
Radioactive or luminescent DNA probes that contain sequences complementary to the STRs attach by base pairing to specific parts of the fragments.
Any unbound probes are washed off.
A film sensitive to X-rays or the wavelengths emitted by luminescent probes is placed over the Southern blot overnight.
The film is exposed and the autoradiograph reveals a banding pattern in which the dark bands show the position of the probe, and therefore the repeated sequences.
This pattern is the genetic fingerprint.
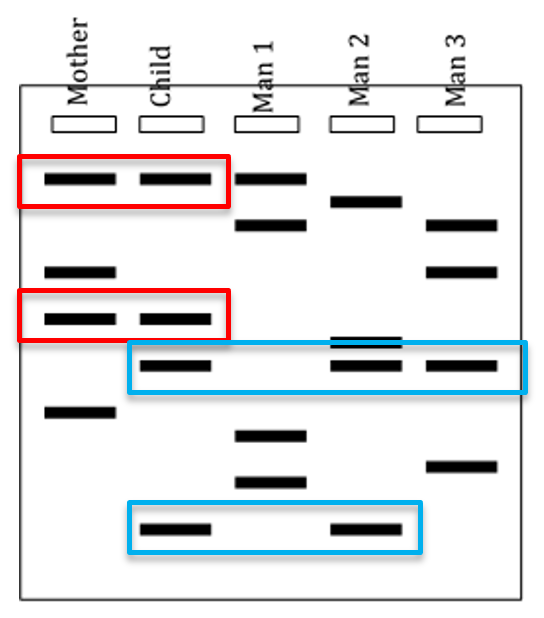
Uses of DNA profiling
This technique was invented by Professor Sir Jefferies.
It is used in:
Paternity - Where the DNA from white blood cells is used to construct DNA profiles.
These are compared to the mother, and any bands they share were inherited from her, and the remaining bands must be from the father.
If they match, there is a high probability it is the father, but it is not absolute proof.
Distinguishing twin type - MZ twins have identical banding patterns, while DZ do not.
Siblings - Adoptees can confirm alleged biological siblings.
If they are blood relatives, their DNA profiles will show many similarities, on average half their genes will be identical.
Immigration - Some visa applications require proof of relation.
Forensic use - To identify and rule out suspects in criminal cases.
Phylogenetic studies - Profiles of members of different taxa can be compared to determine if they were classified suitably and how close their genetic relationship is.
Pros v cons
It does not require an invasive technique such as a blood test, as mouth swabs, hair or urine can be used.
This can be used on samples too small for blood testing.
It has reversed wrongful convictions when used with other tools and evidence.
It can rule out non-matches during police investigations.
Genetic material of people around the world can be stored before groups intermix.
Some people consider asking for a DNA sample as a violation of the right to privacy and civil liberties.
DNA profiles are held in DNA databases, which are vulnerable to misuse and hacking, which could cause a loss of privacy.
Profiles cannot give absolutes, only probabilities. Other testing is needed for this.
Access to and use of data needs regulation, as insurers and employers could use this in their decisions, and private information could be made public.
DNA profiling could cause wrongful convictions if:
The results are not properly explained to juries and judges, who can use it to bias a decision.
Errors may occur in the procedure.
Experimenters are untrustworthy.
DNA evidence is planted at a crime scene.
Genetic engineering
This is the process of manipulating, altering and transferring genes from one organism or species to another, making a genetically modified (GM) organism.
This can include transfer of gene and gene fragments into:
Bacteria to make useful products, such as insulin.
Plants and animals to acquire new characteristics, such as disease resistance.
Humans, to reduce the effects of genetic disease such as Duchenne muscular dystrophy.
When genetic material from two different species is combined, the result is recombinant DNA, so this is sometimes called recombinant DNA technology.
Organisms that have DNA from another species introduced into their cells are known as transgenic organisms.
Organisms that have had their genes altered are genetically modified, but not transgenic as they have no foreign genes.
Introduced DNA is known as donor DNA, and the organism it is introduced to is the host.
When a cell has incorporated a plasmid containing the foreign gene, it is known as transformed.
The process has 5 steps; isolation, insertion, transference, identification and cloning.
Identification and isolation
The example used throughout is the production of human insulin in E.coli bacteria. This has been used since 1978.
Locating the gene
The donor molecule of human DNA contains the gene for insulin.
This is hard to locate, but is done using a gene probe.
This is a specific segment of single-stranded DNA which is complementary to a section of a gene.
Isolating the gene
This can be done using two enzymes, either restriction endonuclease or reverse transcriptase.
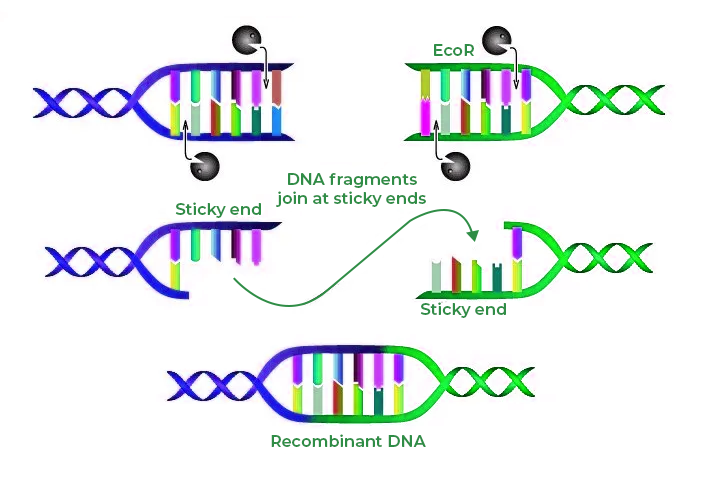
Restriction endonuclease
These enzymes cut DNA at specific nucleotide sequences.
As sequences occur in many places, DNA is cut into many small fragments and individual genes can be isolated.
Some restriction enzymes cut straight across a DNA double helix, a blunt cut.
Many others make a staggered cut, which leaves unpaired bases on both strands.
These pair with complementary sequences very readily, so they are called sticky ends.
E.coli produces a restriction endonuclease called EcoR1.
This is because it was the first to be isolated, and came from E.coli strain RY13.
This catalyses the formation of breaks in the DNA backbone in a specific sequence of nucleotides, where a nucleotide containing guanine is next to one containing adenine.
The line of cut is staggered, leaving sticky ends when the strands are separated
The four unpaired bases at the end of each of the two strands are in reverse order, forming a palindrome (it reads the same backwards as forwards).
This is also known as a recognition sequence.
If the cut was made either side of the insulin gene, it would be isolated from the rest of the genome.
There are two main drawbacks:
If the recognition sequence occurs within the gene of interest, it will be broken into fragments that have no function.
Eukaryotic genes contain introns, which have no contribution to mRNA coding for polypeptides.
Introns are removed normally from RNA transcripts in the nucleus, before the mature mRNA moves to the cytoplasm.
By using the whole gene, however, introns are present and will be incorporated into the plasmids.
Bacteria are prokaryotes, and will therefore be unable to remove introns as they do not have the appropriate enzymes to remove them after transcription.
Any protein translated will contain extra amino acids representing the intron sequences, and will not be functional.
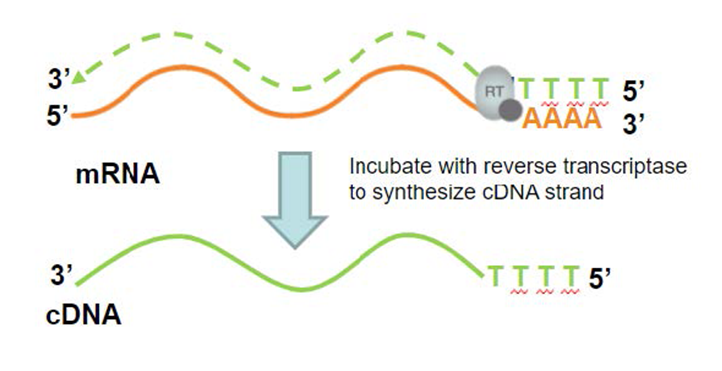
Reverse transcriptase
A cell contains only 2 copies of the insulin gene, but it may contain many molecules of mRNA that have been transcribed from it.
This is especially true for cells that synthesise and secrete this product, such as the cytoplasm of B-cells of the pancreas as it transcribes lots of insulin.
This mRNA can be extracted.
This enzyme produces DNA from an RNA template, and is made by a group of viruses known as retroviruses.
The DNA it synthesises is known as copy DNA or cDNA, which is complementary to the RNA.
DNA polymerase is then used to catalyse the synthesis of a complementary strand of DNA, making a double-stranded molecule containing the insulin gene.
This does not have the issue of introns as the mRNA in the cytoplasm already has had it’s introns removed.
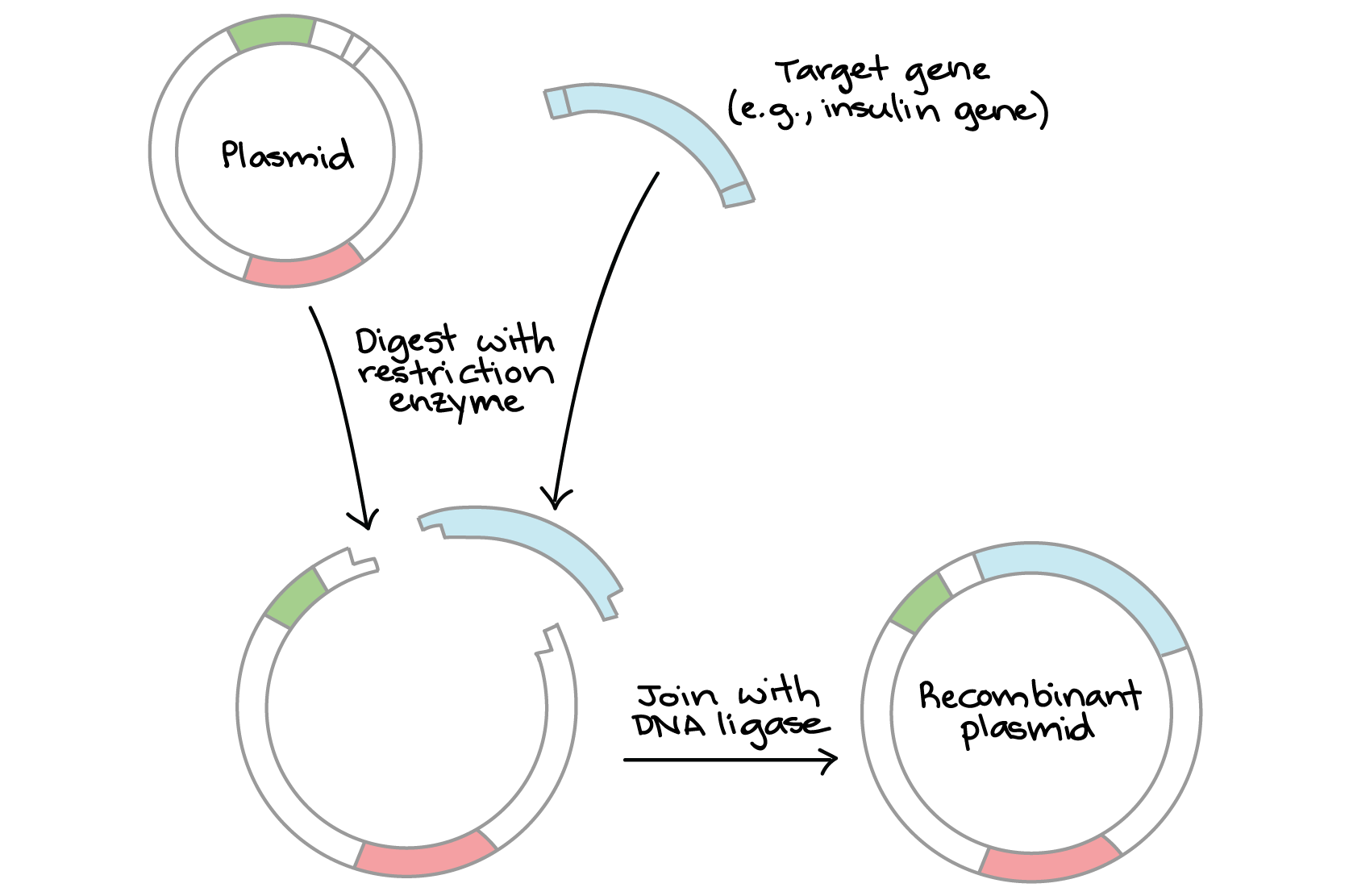
Making a recombinant plasmid
Genes must be carried into the cells by a vector, as bacterial cells are unlikely to spontaneously take up genes.
Vectors are viruses or plasmids used as a vehicle for carrying foreign genetic material into a cell.
In this case, plasmids are used. They are much smaller than viruses, and contain only a few genes.
They can also move in or out of cells.
They are isolated using:
EDTA to destabilise cell walls.
Detergent to dissolve the phospholipid cell membrane.
Sodium hydroxide to make an alkaline environment that denatures the membrane proteins.
Plasmids are then separated from the cell debris.
Restriction endonuclease is then used to cut open the circular DNA molecule making up the plasmid.
This means it has the same nucleotide sequences in its sticky ends.
The vector and the gene are mixed and their complementary base sequences pair with each other.
The gene is now loosely bound to the plasmid, and DNA ligase is used to make the join permanent.
It bonds molecules together, in this case the sugar-phosphate backbones of the gene and the plasmid.
The gene has now been ‘spliced‘ into the vector and is now recombinant DNA.
Good vectors are:
Self-replicating.
Small.
Not broken down by enzymes of the host cell.
Do not stimulate immune responses.
Screenable to prove the gene was actually inserted into the vector.
Have markers which allow successful host cells to be identified.
Transfer into host cell
As few as 1% of bacterial cells take up the plasmid and are transformed.
This can be increased by using calcium chloride, which has a positive charge on the calcium ions.
This binds the negatively charged DNA backbone of the plasmid and the membrane lipopolysaccharides.
The DNA plasmid passes into the cells with a heat shock, where cells chilled to 4 degrees are briefly heated to 42 degrees.
Genetic markers
To produce a successful host, the plasmid must successfully incorporate the gene, and the bacteria must take up the plasmid.
This can be confirmed via:
Vectors that have not taken up the gene of interest are known as empty, and identified by DNA sequencing.
Plasmids used have antibiotic-resistant genes - which can range from ampicillin, tetracycline and chloramphenicol.
Cells are cultured on a growth medium containing the antibiotic.
If the plasmid was successfully incorporated, they will not be broken down by the antibiotic and grow.
If they do not contain the plasmid, they will be killed by the antibiotic.
This antibiotic resistance gene is known as a marker gene, as it distinguishes the plasmids presence.
To distinguish cells that have taken up empty plasmids, blue-white screening is used.
This grows cells on a medium containing lactose analogue, X-gal.
They turn white if they contain a plasmid with the gene, but blue if the plasmid is empty.
Bacterial cells with recombinant plasmids are then cultured in large volumes in fermenters.
Each culture forms a clone, which produces multiple copies.
Each bacterial cell contains around 40 plasmids, and when cells replicate, so do plasmids.
Bacterial enzymes transcribe the insulin gene in the plasmid, and translate the mRNA they produce.
Insulin is made in large quantities and then purified for medical usage.
Pros of GM bacteria
This process has been hugely beneficial in:
Medical products - large amounts of pure human proteins for medical usage have been made, such as insulin, clotting factors for haemophiliacs, human growth hormones for dwarfism, etc.
These are safer than older products which were purified from cadavers and could transmit disease, such as clotting factors causing HIV-AIDS and hepatitis C.
Tooth decay - The oral bacterium Streptococcus mutans makes lactic acid, and is a major contributor to tooth decay.
Modified strains that do not produce lactic acid are available, which out-compete the original S. mutans in the mouth and reduce cavity formation.
Prevention and disease treatment - Bacteria have been modified to produce vaccines and treat disease, such as to hinder tumours and fight Crohn’s disease.
Enhancement of crop growth - Modified bacteria can secrete molecules toxic to plant pests.
Environmental use - Detecting and removing environmental hazards can be done via modified bacteria.
Modified bacteria have been used to clean up mercury pollution and detected arsenic in drinking water.
Cons of GM bacteria
Plasmids are easily transferable, and DNA is often exchanged between bacteria.
This means the antibiotic resistance genes can be transferred, and potentially taken up by pathogenic species.
Fragments of human DNA used to make gene samples or cytoplasmic mRNA used to make cDNA may contain oncogenes or gene switches that activate proto-oncogenes.
There must be protocols in place to ensure these do not contaminate the final product.
A micro-organism with a new gene may become a threat is released into the environment.
A newly introduced gene may disrupt the normal function of genes in ways not understood.
Genetically modified crops
Human population has grown over time, and alongside it has our requirement for food.
This is worsened due to meat consumption increasing alongside affluence, as the lower the trophic level food is consumed the more efficient.
Additionally, the meat industry is an inefficient user of water, land and food.
It is estimated that 70% of plants are lost between harvesting and supplying, and GM plants have been suggested to counter this.
There are many ways of introducing a novel gene into plants:
A ‘gene gun‘, which fires small spheres, normally gold or tungsten, coated with a preparation of the gene at plant cells.
Some penetrate the cell wall and are taken up through the cell membrane.
Electroporation, where an electric field is used to increase the permeability of cell membranes, enhancing gene uptake.
Microinjection, where a membrane is pierced by an ultra-fine needle and the gene is injected into the cytoplasm or nucleus.
This is much more developed for use in animal cells compared to plants.
A bacterial vector, Agrobacterium tumefaciens. This is the most common method for making transgenic plant cells.
A. tumefaciens
This is a soil bacteria which infects plants. A section of the bacterium’s plasmid known as T-DNA, can integrate into the plant’s chromosomes.
The plasmid genes are then transcribed and translated, and the auxins (plant hormones) they produce cause a tumour, or gall, to form, giving the plant crown gall disease.
This plasmid is known as the Ti (tumour inducing) plasmid.
By splicing chosen genes into the plasmid, these genes can be transferred into plant cells, which are then grown in tissue culture and regenerated into plants which express these introduced genes.
Soya beans
In the UK, these make up around 60% of manufactured foods.
They are routinely treated with herbicides, but can damage the plants, meaning they cannot be treated while growing.
‘Roundup Ready‘ soybeans, grown commercially since 1996, are genetically modified to contain a gene that makes them herbicide resistant.
These crops can therefore be sprayed without disturbing their growth.
Tomatoes
Bt tomatoes
Bacillus thuringiensis is a bacterium which lives in the soil, and contains a plasmid which codes for a protein that can act as an insecticide.
This has been incorporated into the cells of these tomatoes.
These proteins are produced in leaves, avoiding the fruits which humans consume.
These reduces costs associated with insecticides, and potential damages caused by insecticides.
These tomatoes have been given resistance to:
Tobacco hornworm (Manduca sexta).
Tomato fruitworm (Heliothis zea).
Tomato pinworm (Keiferia lycopersicella).
Tomato fruit borer (Helicoverpa armigera).
A 91 day feeding trial on rats showed no adverse effects, but they have never been commercialised.
Antisense tomatoes
Tomatoes ripen due to the enzyme polygalacturonase, which breaks down the pectin in their cell walls.
If transported long distances, this enzyme can cause them to over ripen and become un-sellable.
The ‘Flavr Savr‘ tomato was grown commercially from 1994-1997 to overcome this.
A. tumefaciens was used to introduce a second copy of the polygalacturonase enzyme, which had a complementary base sequence to the normal gene, making it an antisense gene.
The mRNA transcribed from this gene is therefore complementary to the mRNA strand synthesised from the original gene.
They then pair in the cytoplasm, forming a double stranded molecule and preventing the mRNA of the original gene to be translated, blocking polygalacturonase production.
This gives these tomatoes a longer shelf life.
Traditional plant breeding techniques of hybridisation and crossing improved their flavour, but they still failed to be a commercial success.
Benefits of GM crops
Higher crop yield - Genes that provide natural insecticides, pesticides and weather resistance (drought and salt) will be more likely to survive, therefore increasing yield.
Pesticide reduction - Pesticide genes will remove the need for these, increasing profit and decreasing damage to the environment.
Improved quality - Nutritional qualities of food can be enhanced, such as with Golden Rice, which has been modified to increase vitamin A precursors and could help to prevent blindness if licensed.
Food can also have improved flavour and shelf life.
‘Pharming‘ - This is the practise of producing pharmaceutical molecules in GM crop plants, such as antibodies, blood products, hormones, recombinant enzymes and human and animal vaccines.
Nitrogen - Attempts are being made to make transgenic cereals which contain the nif (nitrogen-fixing) genes from nitrogen-fixing bacteria
They would then have their own nitrogen, and would require less fertilisers, reducing environmental damage and profits.
Negatives of GM crops
Pollen - This could cause GM genes to spread to wild plants, and producing what are known as ‘superweeds‘.
It could also spread to organic crops, compromising them.
Pest resistance - Resistance in plants to pests could cause a population of resistant insects or fungi.
Field trials are needed to determine if this is a well founded concern.
This could be countered by making GM plants resistance to multiple pests, resistance to both is less likely to develop.
Marker genes - In testing, some marker genes contain antibiotic resistance. These genes could potentially be transferred to gut bacteria in consumers.
Plant breeding - This may become controlled by a few companies, limiting the varieties available to farmers and potentially eliminating old ones.
This will reduce biodiversity, and crop gene pools.
New proteins - There is concern that as GM crops produce new proteins due to new genes, these may be harmful to the consumer.
Extensive GM research has been unable to provide any evidence for this claim.
Economics - GM crops are subject to intellectual property laws, and farmers may have to pay for this.
Consequences
These controversies have led to litigation, international trade disputes, protests and strict legislation banning GM crops.
Manufacturers and supermarkets have banned GM ingredients.
GM plants are grown throughout the world, but not in the UK.
Although the UK imports GM animal feed and products, such as maize and soya, to be incorporated in processed food.
Genetic screening
Genetic screening can be done by patients and their families to discover:
Confirm a diagnosis.
Decide appropriate treatment.
Identify people at high risk for preventable conditions.
The inheritable nature of a disorder.
People have concerns about:
It can be seen as an invasion of privacy.
This does not matter if consent is obtained.
Defective alleles being identified prenatally could increase the number of abortions.
Individuals identified as having genetic defects may be placed in high-risk insurance groups, making this expensive or impossible to obtain.
This is the UK - we don’t need medical insurance.
There are many types of genetic testing used in hospitals:
Carrier screening to check for carried recessive alleles.
Pre-implantation testing for IVF embryos.
Prenatal testing.
Newborn baby screening.
Pre-symptomatic testing for adult-onset disorders, such as cancer and Alzheimer’s disease.
Confirming a diagnosis.
Forensic and identity testing.
Some are also commercially offered, and give probabilities of developing certain conditions as well as drug metabolisms. Issues include:
They are not regulated or independently verified.
Only test a small number of genes.
Positive results are not guarantees of developing the disease.
Lab errors can occur.
No available medical options for treatment.
Can cause anxiety.
Then don’t buy one.
Could cause discrimination.
Don’t tell people your results?
Gene therapy
Some genetic disease can be treated by replicating the function of genes.
Gene therapy is when defective alleles are replaced by ones cloned from healthy individuals, which could cure or alleviate symptoms.
To deliver these therapies, certain areas must be targeted using:
Virus as a vector.
Plasmid as a vector.
Injection of naked (no vector) plasmid DNA.
There are also two types of this therapy:
Somatic cell therapy - Targets body cells in affected tissues, which means changes are not inherited in future generations.
Germ-line therapy - Introduces corrective genes into germ-line cells - the oocyte - so the change is inherited.
This is controversial, as genes can interact with each other in unknown ways and affect future generations negatively.
Duchenne muscular dystrophy (DMD)
This is a recessive, sex-linked form of muscular dystrophy.
This is mostly caused by one or more deletions in the dystrophin (stabilises muscle contractions to prevent damage) gene.
This gene has 79 exons, and deletions in any part alter the reading of the gene.
The ribosome meets a stop codon too soon, so the protein is not introduced.
This means a removal of exon 39 would render every exon after it unreadable.
This can cause severe muscle loss, and most sufferers are wheelchair bound by the time they are teenagers.
Life expectancy is 27.
The drug drisaperson is an antisense oligonucleotide - a 50 nucleotide sequence that is complementary to the mutated sequence.
It treats DMD by acting as a molecular patch - binding to the mRNA over the exon with the deletion.
That portion therefore becomes double stranded, and RNA cannot translate it.
This restores the reading frame, allowing for a shorter and partially functional version of the gene to be synthesised.
This is known as exon skipping.
Drisapersen is delivered via subcutaneous injections.
Clinical trials have not yet provided clear evidence to the best age or length of treatment. Other treatments include.
Gene therapy via inserted a shortened version of the healthy gene, as the normal gene is too big to fit in a virus.
Stem cell research.
Cystic fibrosis (CF)
Those with CF are homozygous for the recessive allele.
The normal allele, the cystic fibrosis transmembrane regulator (CFTR) codes for a protein that transports chloride ions out of cells.
Sodium ions follow, and water then leaves by osmosis.
This makes extracellular mucus watery.
The mutant allele cannot transport ions, and this causes thick and sticky mucus which:
Causes bronchioles and alveoli to become clogged, causing congestion and difficulty in breathing.
Mucus is hard to remove, causing recurrent infections.
To keep airways open, chest physiotherapy is required.
Pancreatic duct becomes blocked, stopping pancreatic enzymes from reaching the duodenum.
This prevents digestion and limits absorption.
Children with CF often have large appetites to compensate.
The vas deferens in males can become blocked, causing infertility.
The CFTR gene has been isolated and cloned.
Liposomes, which are hollow phospholipid spheres containing gene preparation are the most successful method of transport.
They are inhaled via an inhaler and fuse with the phospholipid bilayer of the lung epithelial cell membranes.
DNA enters the cells, which transcribe the inserted gene and make the CFTR protein.
This remains functional, but once the cells are replaced, the treatment must be repeated.
Another approach is to use a drug, ivacaftor, which corrects the protein folding.
This can only be done where the specific CF mutation causes a misfolded CFTR protein.
Genomics and healthcare
Genomics is a field which analyses the structure and functioning of genomes, and can be applied to medicine, biotechnology, genetic mapping and bioinformatics.
DNA is annotated, meaning base sequences are used to predict whether sequences code for RNA, proteins, or have a regulatory function.
This can infer what metabolic pathways are controlled, and genomes can be compared.
Healthcare improvements are anticipated, such as:
More accurate diagnoses:
The 100K project analysed the genomes of two brothers with muscle loss and weakness called peripheral neuropathy.
They found the brothers had a new mutation, allowing them to join treatment trials to benefit other members of their family with the same mutation.
Predicting the effects of drugs:
Codeine is converted into morphine, which is detoxified and excreted.
For those which too much morphine is produced or excretion is impaired, normal codeine doses can be toxic.
Genomic information can show metabolic pathways, which can inform a decision about prescriptions.
New and improved treatments:
Testing a tumour can identify genetic changes for which a specific drug is beneficial.
Dosages:
NGS technology sequences genes quickly which can allow patients to have individual treatments based on the DNA.
Warfarin is a common anticoagulant which prevents strokes and blood clots.
Dosages can be complex due to the amount of factors involved, drug interactions, age, genetic factors, etc.
Genetic testing can identify two gene variants, CYP2C9 and VKORC1 based on the genotype a patient has.
Tissue engineering
This uses biochemical, biological, engineering and material science methods to repair, improve or replace biological functions.
This has allowed for the replacement of many tissues and organs, such as trachea, bone, bladder and skin.
The first artificial skin, called Apligraf, was licensed in 1998 and is widely used for burn patients in place of skin grafts.
Artificial systems that mimic the pancreas and liver have been constructed.
Artificial meat is also hoped to be made using these techniques, along with treatments of diabetes, nerve tissue, hearing and vision loss and genetic disorders.
Cells for tissue engineering
Telomeres are the repeated nucleotide sequences on the end of chromosomes, which shorten at each division and limit cell lifespan.
In 1998, a technique was developed for elongating this, extending the lifespan of differentiated cells.
Cells can be separated from tissues with enzymes, such as trypsin, and undergo telomere elongation in order to be used to grow tissue replacements, such as chondrocytes for cartilage replacements.
Stem cells are still preferred.
Cells for tissue engineering are classified by source:
Autologous cells are from the same individual.
They have less problems with rejection and pathogen transmission, but are not always available if a patient has a genetic disease or another weakness.
Allogenic cells are from a donor of the same species.
Xenogenic cells are from another species.
Pig cells are used to develop cardiovascular implants.
However, there is potential they contain viral sequences which are harmful to humans, but it is claimed these sequences are removed.
Syngenic or isogenic cells are from genetically identical organisms.
Scaffolds
This is when cells are ‘seeded‘ onto a scaffold - an artificial structure that can support a 3D tissue.
It must:
Allow cells to attach and move.
Deliver and retain cells and biological molecules.
Be porous to allow diffuse of nutrients and waste products.
Be biodegradable and absorbed by surrounding tissues.
The rate it degrades should match the rate of tissue formation, so it will eventually break down leaving neotissue.
Tissue culture
Cells grown in tissue culture form cell lines (animal cells that can be grown indefinitely) that are clones, as all the cells derived from one parent are genetically identical.
This can be used to produce cloned tissues or organs.
This is known as therapeutic cloning, the opposite of growing whole organisms such as reproductive cloning.
Only the former is legal.
During culturing, optimum conditions are required, and as structures grow larger other mechanisms such as capillaries are needed for diffusion.
Sometimes cells require special physical or chemical structures, such as chondrocytes needing low oxygen to mimic development in skeletal tissue.
Stem cells
These are unspecialised cells, which can differentiate into many possible cells.
Types
Embryonic stem cells (ESCs) are found in 3-5 day old embryos. They are in blastocysts, and can differentiate into all cells.
They are described as totipotent, able to differentiate into every cell and develop a whole organism.
Some adult tissues contain stem cells, contained in a special area known as a stem cell niche.
They can replace cells lost via normal wear and tear, injury or disease, but cannot form all cell types.
In some organs, such as the heart, stem cells only divide under certain conditions.
Reprogrammed adult stem cells were developed in 2006, which were reprogrammed to be induced pluripotent (iPSCs), which behave as ESCs.
Pluripotent means they can develop into many different cell types, but not an entire organism on their own.
Potential
They can be used in tissue engineering to regenerate tissues and organs, such as insulin-producing cells to cure diabetes 1.
Cell-based therapies for disease treatment. They can be used as replacement cells and tissues to treat diseases, which is especially important due to the high need for transplantable tissues.
Drug screening. Cancer cell lines have been used to screen for anti-tumour drugs but stem cells allow for many other tissues to be tested.
Development of model system for growth, which could help identify the causes of birth defects.
To investigate the events that occur during human development, and how gene switches turn undifferentiated cells into differentiated cells.
Comparison
Embryonic stem cells can become any cell type, while adult stem cells are more limited.
Blastocysts may contain about 100 ESCs, so they can be isolated in useful numbers.
Adults stem cells are rare, which makes them difficult to isolate.
Embryonic stem cells are easily cultured and large quantities are readily produced, whereas techniques for culturing adult stem cells are less developed.
Pros v cons
Stem cells will make the issue of organ shortages less significant.
ESC-made tissue requires less immunosuppressants, and cells derived from a patient’s own stem cells are less likely to provoke immune rejection.
Techniques for extracting, culturing and manipulating stem cells are still in development and the behaviour of cell structures can be unpredictable.
Stem cell technology is therefore quite rare.
Stem cell technology is quite new, meaning long-term studies have not been conducted.
There are concerns relating to premature cell aging and other unpredictable events.
Ethical issues
ESCs
The Human Fertilisation and Embryology Authority (HFEA) grants licences on embryo research for up to 14 days after fertilisation. They require:
Stem cell lines or cell lines are maintained indefinitely and may be used in many different research projects or clinical therapy.
Stem cells are deposited in the UK Stem Cell Bank so they are available to other research groups, both nationally and internationally.
There is no financial reward for any development or discovery made using them, although they can’t be pirated.
Donors must give specific consent to embryos created with their gametes being used in stem cells research.
There are concerns around:
The source of ESCs - Recently it has been allowed for researchers to create embryos for research, but previously only spare IVF embryos could be used.
Some argue that creating embryos specifically for research is against human life, as it is created as a means to an end.
However, these embryos could never legally be transferred to a uterus, so they could never be a human.
Moral status of an embryo - Under the Human Fertilisation and Embryology Act an embryo does have moral rights, but not equivalent to a human being.
Groups that believe life begins at conceptions believe they should have full human rights.
Others believe embryos gain human rights the further along development you move.
The rights of a foetus should be balanced against the benefits that people may gain from ESC research.
Some argue it is never justified due to adult stem cells and iPACs.
However, researchers argue ESCs are still important due to their ability to:
Clarify fundamental biological mechanisms.
They will indicate which types of stem cells will be most useful in cell-based treatments.
A pre-14 day old embryo is a ball or cells with no possibility of human existence, so it’s usage is justified.
Some people fear this could lead to reproductive cloning, which is illegal in the UK.
This can be seen as devaluing human life.
Adult stem cells
Adult stem cells have been used in research and medicine for many years, such as bone marrow transplants for leukemia. There is no ethical issues surrounding their usage.