004 General Biology 1.docx
[MODULE 1] BIOMOLECULES: CARBOHYDRATES AND LIPIDS
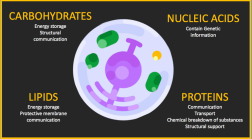
BIOMOLECULES
• Major categories of Biomolecules:
Carbohydrates, Lipids, Proteins, Nucleic Acid
• All biomolecules are composed of monomer and polymer.
How are they formed?
Dehydration Synthesis and Hydrolysis Reaction
Polymer | Monomer | |
|---|---|---|
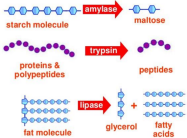
Carbohydrates | Polysaccharides (Starch, Glycogen, etc.) | Monosaccharides (Glucose, Fructose, etc.) |
Lipids | Fats(Triglycerides, Oils, Waxes) | Triglycerides :Glycerol and Fatty Acids |
Proteins | Protein; Polypeptides | Amino Acids |
Nucleic Acid | DNA & RNA | Nucleotides(GCAT and GCAU) |
CARBOHYDRATES
• Carbon, Hydrogen, and Oxygen: CnH2nOn
Carbohydrates are sources of energy
The smallest carbohydrate is called monosaccharide, while the complex carbohydrate is polysaccharides.
All carbohydrates have approximately 2 hydrogen atoms and 1 oxygen atom (e.g., water) for each carbon atom, hence the name ‘hydrates of carbon.’
MONOSACCHARIDES - Building block of carbohydrates.
Monosaccharides - Major cellular nutrient.
Monosaccharides - Often incorporated into more complex carbohydrates.
Monosaccharides - Can be converted into other organic molecules. Example: Glucose, Galactose, Fructose
• Mnemonics: GGF (Gives Good Flavor)
DISACCHARIDES are an Energy source.
Disaccharides are sweeteners and dietary components.
Disaccharides are composed of two or monomers joined by a glycosidic bond • Example: Lactose (galactose + glucose), Sucrose (glucose + fructose), Maltose (glucose + glucose*)*
• Mnemonics: LSM (Length Supports Movement)
POLYSACCHARIDES Consist of hundreds of linked monosaccharides
Polysaccharide are complex carbohydrates
• Storage and structural material
• Example: Cellulose, Glycogen, Starch
• Mnemonics: CGS (Can Get Stored)
GLYCOSIDIC BOND - connects one (1) monosaccharide to another because of the dehydration synthesis or the condensation reaction
• Simple carbs (simple sugars) are found in most candy and sweet drinks, fruits, vegetables, and milk. They are quick to digest and give short burst of energy.
• Complex carbs (like starches) are found in pasta, bread, potatoes, legumes, and corn. They take longer to digest and prove energy longer.
• All lipids are insoluble in water. Emulsifiers allow lipids to mix in water.
Lipids are hydrophobic.
Lipids have extremely diverse chemical structures. • They are classified to differences in structure and function
Fats – energy storage molecules, insulators against heat loss, and cushion tissue for organs. o Oils – are generally something in our diet, however they are converted to fats in our bodies and therefore only function as a nutrient.
Phospholipids – main component of membranes. o Steroids – hormones (messenger molecules) and are components of cell membranes (cholesterol)
TRIGLYCERIDES OR FATS - Energy storage and insulations
• Cushioning of vital organs
• All formed from one glycerol molecule reacted with three fatty acid molecules through a condensation synthesis reaction.
• Made out of 2 basic units (building blocks):
o Glycerol (alcohol) – is a three (3) carbon molecule that formed the backbone of triglyceride
o Fatty Acids – consists of three (3) long hydrocarbon chains that divides these fats into two groups:
▪ Saturated Fatty Acid – no double bonds,
solid (at room temp)
▪ Unsaturated Fatty Acids – one or more
double bonds, liquid (at room temp)
TYPES OF FATS IN OILS |
|---|
SATURATED |
• Chain of carbon atoms “saturated” with hydrogen• Solid at room temp• High melting point |
POLYUNSATURATED |
• Chain of carbon atoms with multiple double bonds• Liquid at room temp |
• Lowest melting point | • Liquid oils industrially converted into solids• High melting points |
|---|
PROPERTIES OF FATS AND OILS
Oil – a mixture of triacylglycerols that is liquid because it contains a high proportion of unsaturated fatty acids.
Fat – a mixture of triacylglycerols that is solid because it contains a high proportion of saturated fatty acids
PHOSPHOLIPIDS - Major component of cell membranes.
A cell membrane is made up of two phospholipids;
Polar head (hydrophilic) – organic molecule (e.g. choline) and a phosphate group
Non-polar tail (hydrophobic) – Diglyceride: glycerol + 2 fatty acids
The phosphate/Nitrogen group is charge and phospholipids therefore have non-polar hydrophobic regions (fatty acids and the glycerol) and polar hydrophilic regions (the phosphate/nitrogen group).
STEROIDS
• Structure: four interconnected carbon rings. (ex. vitamin D,
cortisone, cholesterol)
Steroids have two principal biological functions: as
important components of cell membranes which alter
membrane fluidity; and as signaling molecules.
Steroids are base of sex hormones
Steroids are emulsification of fats during digestion
Good Cholesterol (High-Density Lipoprotein) – carries cholesterol from other parts of your body back to your liver.
Bad Cholesterol (Low-Density Lipoprotein) – transports cholesterol from the liver to the tissues of the body.
HEALTH FACTS
• Saturated fats are associated with heart disease
• Fatty acids promote higher levels of blood cholesterol
• Animal fats also contain cholesterol, plants have no
cholesterol
[MODULE 2] BIOMOLECULES: PROTEINS AND NUCLEIC ACIDS; ENYZMES
PROTEINS
• Protein is an energy-yielding nutrient composed of carbon, hydrogen, oxygen, and nitrogen.
• Differs from carbohydrates and fats because of the presence of nitrogen.
• The body has at least 30,000 types of protein, each with a different job (many roles)
• The building blocks of all protein molecules are amino acids
• Complex structure
• Energy Source
AMINO ACID - Possess carboxyl and amino groups
Amino acid differ in their properties due to differing side chains (R Groups)
HOW ARE THESE AMINO ACIDS LINKED?
A peptide bond is a chemical bond formed between two molecules when the carboxyl group of one molecule reacts with the amino group of the other molecule, releasing a molecule of water (H2O)
This is a dehydration synthesis reaction (also known as a condensation reaction), and usually occurs between amino acids.
Two linked amino acids forms a dipeptide, three forms a tripeptide, and long chains of amino acids are polypeptide
FOUR LEVELS OF PROTEIN STRUCTURE: Primary Structure - Amino acid sequence, Secondary Structure - Hydrogen bonding, Tertiary Structure - Side chain interactions, Quaternary Structure - 2 of more polypeptides
AMINO ACIDS CAN BE CLASSIFIED IN TWO WAYS:
• Essential Amino Acids
o There are 21 amino acids that are required for building proteins in our bodies.
▪ Essential/Indispensable amino acids must be ingested since our bodies do not manufacture these molecules
▪ 9 amino acids that come from the diet
• Non-Essential Amino Acids
o Non-essential/dispensable amino acids can be made by our bodies from other amino acids.
▪ Your body can synthesize 11 of the
amino acids from the other amino acids
Essential | Conditionally****Non-Essential | Non-Essential |
|---|---|---|
• Histidine• Isoleucine• Leucine• Methionine | • Arganine• Asparagine• Glutamine• Glycine | • Alanine• Asparatate• Cysteine• Glutamine |
K. HERRERA | 12 - COTTAM
• Phenylalanine• Threonine• Tryptophan• Valine• Lysine | • Proline• Serine• Tyrosine(usually, non essential except in times of illness and stress) |
|---|
In eukaryotes, there are only 21 proteinogenic amino acids, the 20 of the standard genetic code, plus selenocysteine.
DISTRIBUTION OF PROTEINS IN THE BODY • Muscle
• Bone
• Skin
• Other: blood, glands, nerve, tissue
PROTEIN FUNCTIONS
• Enzymatic (rubisco)
• Storage (ferritin)
• Structural (spider silk)
• Protection (immunoglobulin)
• Hormonal (insulin)
• Contractile (actin)
• Receptor (rhodopsin)
• Transport (hemoglobin)
STRUCTURAL ROLES
• Keratin
o Makes up hair and nails
• Collagen
o Supports in ligaments, tendons, and skin
• Actin and Myosin
o Make up muscle fibers in muscle cells that allow contraction and are major component of the cytoskeleton of cells.
• Histones
o Protein associated with DNA to make chromosomes.
• Intercellular Filaments
o Hold cells together.
HORMONAL ROLES
• Insulin
o Messenger molecule in blood from pancreas that signals for cells to absorb glucose.
• Cyclin
o Messenger molecule in blood to signal cells to go into stages of mitosis
TRANSPORTATION ROLES
• Hemoglobin
o Transports oxygen to the blood
MODULE 2: BIOMOLECULES: PROTEINS AND NUCLEIC ACIDS; ENZYMES
DENATURATION
• The protein we consume can be altered and changed but can never return to its initial form. This is called denaturation.
• Factors that cause denaturation:
o Heat
o Acids
o Bases
o Alcohol
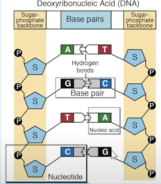
NUCLEIC ACIDS
• Store and transmit hereditary/genetic information • Nucleic acid is a polymer consisting of monomers called the nucleotides and polynucleotides
• Are large organic molecules that carry the “code of life” • 2 main types of nucleic acids:
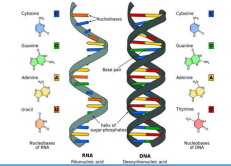
o Deoxyribonucleic acid (DNA) – double helix
o Ribonucleic acid (RNA) – single helix
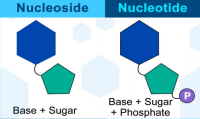
NUCLEOSIDE
• The substructure composed of nucleobase plus sugar; without phosphate
• 4 N-containing bases found in DNA:
o Guanine
o Cytosine
THERE ARE FIVE NITROGENEOUS BASES IN TOTAL 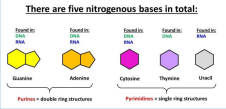
• Unique sequence of nucleotides
• Types:
o Deoxyribonucleic Acid (DNA)
o Ribonucleic Acid (RNA)
DEOXYRIBONUCLEIC ACID
• Stores information for the synthesis of specific proteins • Directs rRNA synthesis
• Deoxyribose
• A, T, C, G
• Forms a double helix
o Adenine
o Thymine
• For RNA, thymine is replaced with Uracil
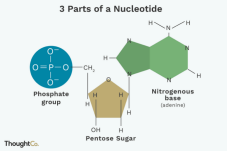
NUCLEOTIDE
• Consists of: Pentose sugar, Nitrogenous base, Phosphate group
RIBONUCLEIC ACID
• Essential in coding, decoding, expression, and regulation of genes
• Single chain
• A, C, G, U
MODULE 2: BIOMOLECULES: PROTEINS AND NUCLEIC ACIDS; ENZYMES
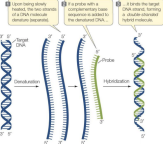
MELTING AND RE-ANNEALING
High temperature and/or low salt concentration causes the two strands to melt or disassociate.
Hybridization: in a mixture of DNA with different sequences, the complementary strands will find each other in the mixture.
JAMES WATSON & FRANCIS CRICK - Describes DNA as a double helical structure ENZYMES AND FACTOR AFFECTING ITS ACTIVITY
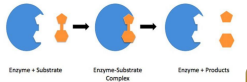
enzymes - It increases the speed of chemical reactions without being consumed by the reaction.
Not all enzymes are proteins. example: Ribozymes – made of RNA
STRUCTURE OF ENZYMES
• Enzyme Structure
o Proteins that work as catalyst
o Speed up chemical reactions without being altered themselves.
MECHANISM
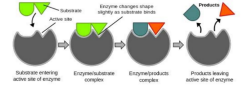
• In most instances, only one small part of the enzymes, called the active site, associates directly with the substrate (s).
METABOLIC PATHWAY
• A series of linked reactions
• Begin with a particular reactant and end with a final product.
SUBSTRATE BINDING
• Binding a substrate induces the conformational changes in enzyme molecule (induced fit model)
• An enzyme-substrate (ES) complex is formed
LOCK AND KEY MODEL
• The lock and key model of enzyme action, proposed earlier this century, proposed that the substrate was simply drawn into a closely matching cleft on the enzyme molecule.
INDUCED FIT MODEL
• The induced fit model is the configurations of both the enzyme and substrate are modified by substrate binding. • This model proposes that the initial interaction between enzyme and substrate is relatively weak, but that these weak interactions rapidly induce conformational changes in the enzyme that strengthen binding.
ENERGY OF ACTIVATION (EA)
• The energy that must be added to cause molecules to react with one another
MODULE 2: BIOMOLECULES: PROTEINS AND NUCLEIC ACIDS; ENZYMES
SUMMARY OF CARBOHYDRATES, NUCLEIC ACIDS,
FACTORS AFFECTING ENZYME ACTIVITY • Substrate Concentration
o Enzyme activity increases as substrate concentration increases.
• Optimal pH
o Each enzyme has an optimal pH at which the reaction rate is highest
• Temperature
o As temperature rises, enzyme activity increases o Denaturation
EXCEPTIONS
• Environmental influence
• Bacteria living in hot springs have enzymes that withstand high temperatures
• Enzyme inhibition
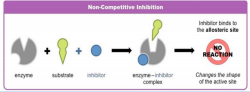
o Occurs when a molecule (the inhibitor) binds to an enzyme and decreases its activity.
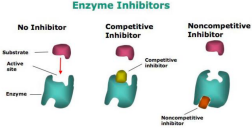
o The inhibitor binds to the enzyme at a location other than the active site known as the allosteric site, thus changing its shape and its function.
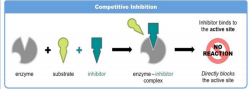
• Competitive inhibition
o Occurs when an inhibitor and the substrate compete for the active site of an enzyme.
LIPIDS, AND PROTEINS