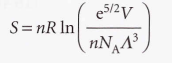Chapter 16 - Statistical Thermodynamics 1: The Concepts
- Statistical thermodynamics - The link between individual molecular properties and bulk thermodynamic properties.
The distribution of molecular states
- Population - The average number of molecules that occupy it.
- Principle of equal a priori probabilities - The assumption that all possibilities for the distribution of energy are equally probable.
16.1 Configurations and weights
Weight of the configuration - The number of ways a general configuration can be achieved.
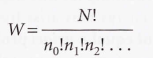
Stirling's approximation
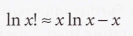
Boltzmann distribution
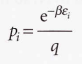
16.2 The molecular partition function
Molecular partition function
Common methods used to reach very low temperatures
- Optical trapping - Where atoms in the gas phase are cooled by inelastic collisions with photons from intense laser beams.
- Adiabatic demagnetization - Based on that, in the absence of a magnetic field, the unpaired electrons of a paramagnetic material are orientated at random.
Thermal wavelength - Decreases with increasing mass and temperature.
The internal energy and the entropy
16.3 The internal energy
The total energy of the system relative to the energy of the lowest state
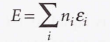
16.4 The statistical entropy
Boltzmann formula for the entropy

The canonical partition function
16.5 The canonical ensemble
Canonical ensemble - The imaginary collection of replications of the actual system with a common temperature.
Microcanonical ensemble - Where the condition of constant temperature is replaced by the requirement that all the systems should have exactly the same energy.
Grand canonical ensemble - The volume and temperature of each system are the same, but they are open, which means that matter can be imagined as able to pass between the systems; the composition of each one may fluctuate, but the chemical potential is the same in each system.
Canonical distribution
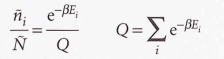
Canonical partition function (Q) - A function of the temperature.
16.6 The thermodynamic information in the partition function
The total weight (W) of a configuration of the ensemble - The product of the average weight W of each member of the ensemble.
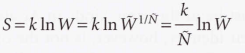
16.7 Independent molecules
For distinguishable independent molecules
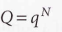
For indistinguishable independent molecules
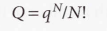
Sackur-Tetrode equation - It implies that the molar entropy of a perfect gas of high molar mass is greater than one of low molar mass under the same conditions. Used for the entropy of a monoatomic gas.