03: Carbohydrate Metabolism: Glycogenesis and Glycogenolysis
Glycogenesis
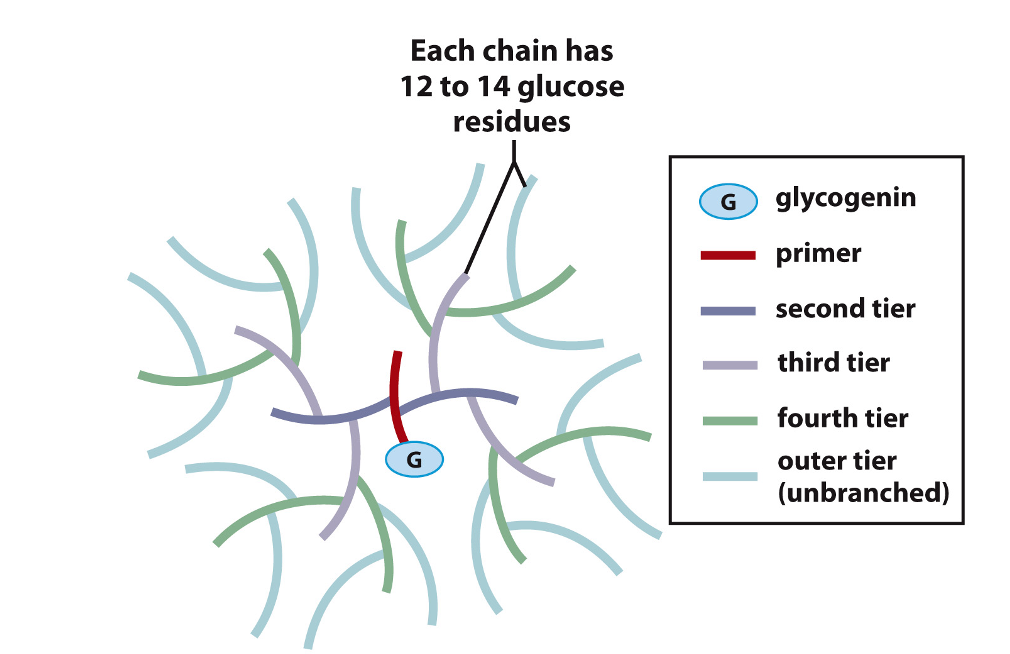
Structure of Glycogen
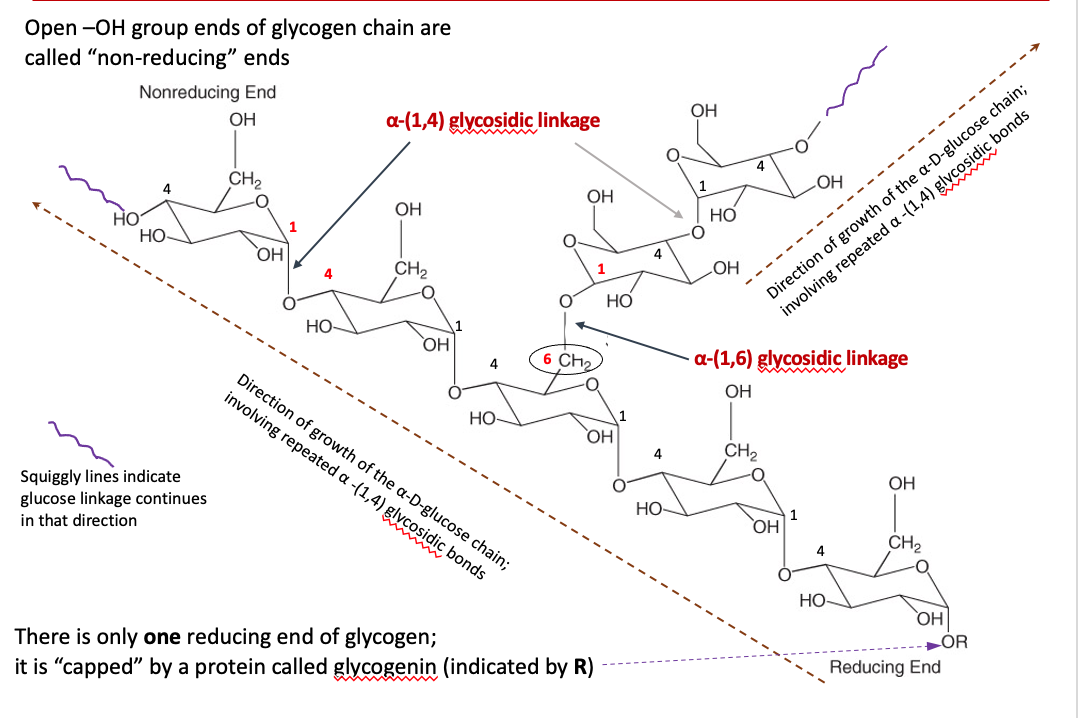
Storage form of glucose; branched polymer
Multiple α–D-glucose units linked by α-(1,4) glycosidic bonds
Linear chain of α–D-glucose
Every 12 or 14 glucose residues, a α-(1,6) bond is created
α-(1,6) bond creates a branch point on the linear chain
Advantages of branching
More glucose units can be “packed” into glycogen’s structure
Prevents crystallization of glucose
General Structure of a Glycogen Particle
Glycogen
Glycogen is the storage form of carbohydrate in animals
Glycogenesis: Synthesis of glycogen from α-D-glucose
Anabolic process (anabolic, catabolic)
Main stores of glycogen: Liver and skeletal muscle
Liver glycogen:
Function: maintain blood glucose concentration
Increase in the well-fed state
Decrease in the starvation state
Muscle glycogen:
Function: fuel reserve for ATP synthesis during exercise
Decreased levels due to strenuous muscle activity
Synthesized when glycogen stores are depleted
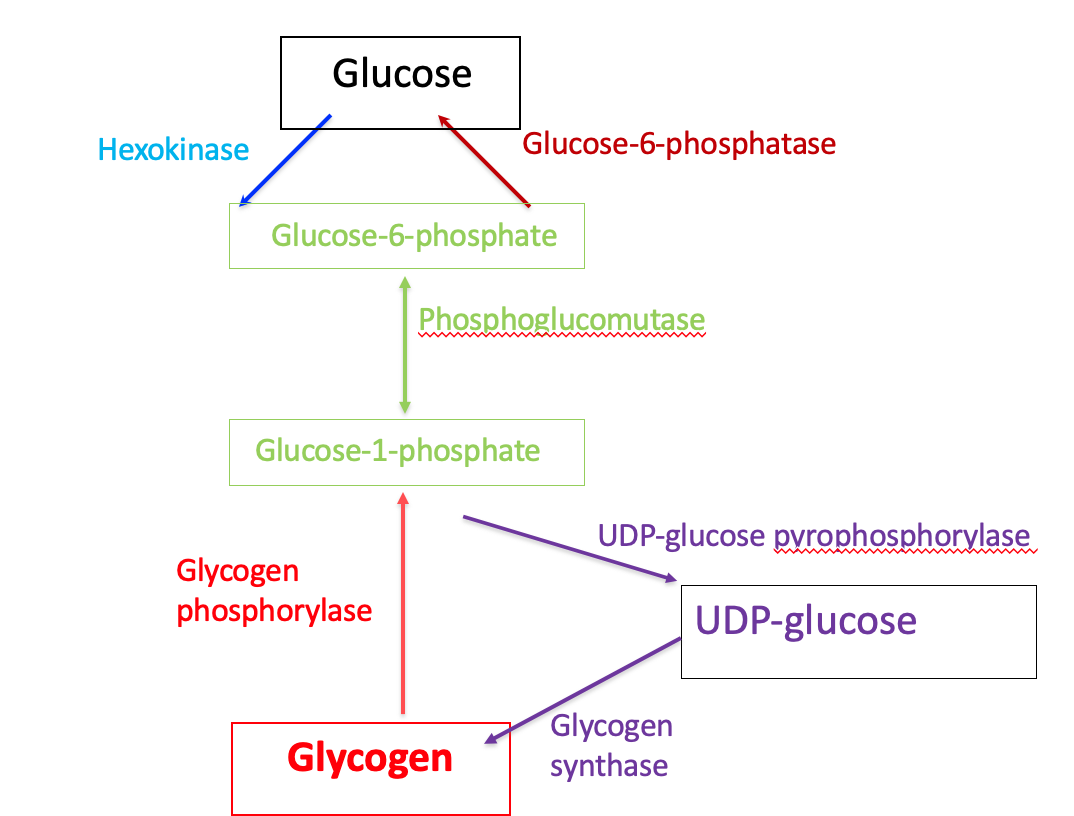
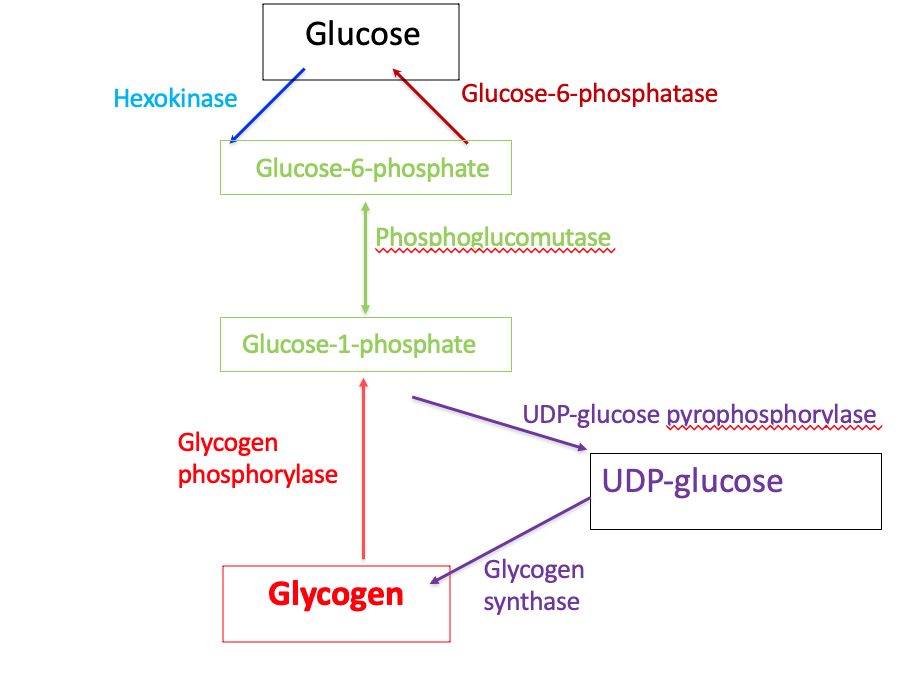
Flow Diagram of Glycogen Metabolism
Glycogenesis: Full Process
step #
action type
enzyme
1
conversion of glucose 6-phosphate to glucose 1-phosphate
phosphoglucomutase
2
synthesis of UDP-glucose
UDP- glucose pyrophoshorylase
3
synthesis of an initiating primer
glycogenin
4
chain elongation
glycogen synthase
5
formation of branches (creation of 1.6 branches and extension of 1,4 branched chain)
4:6 transferase
(branching enzyme)
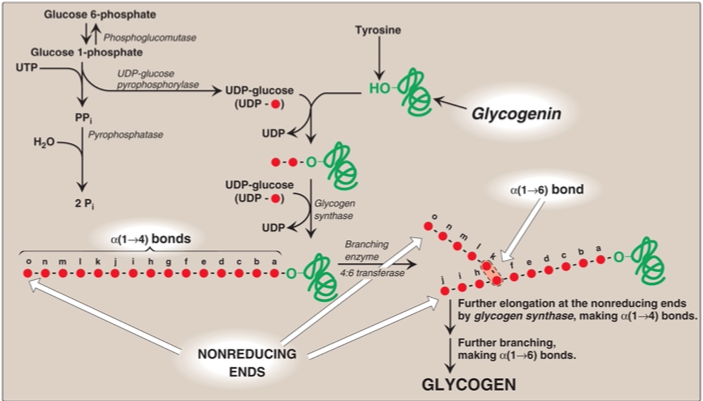
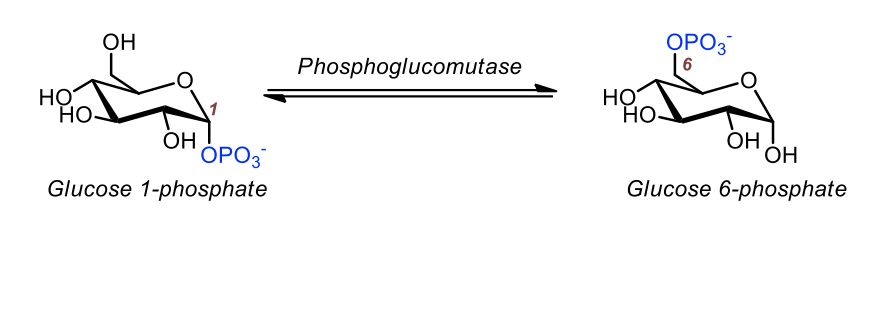
Step 1: conversion of glucose 6-phosphate to glucose 1-phosphate
Glucose 1-phosphate and glucose 6-phosphate: isomers
Interconversion catalyzed by phosphoglucomutase
Step 2: synthesis of UDP-glucose
UDP-glucose is synthesized from glucose 1-phosphate (step 1) and UTP
UDP-glucose pyrophosphorylase hydrolyzes UTP by UDP-pyrophosphorylase
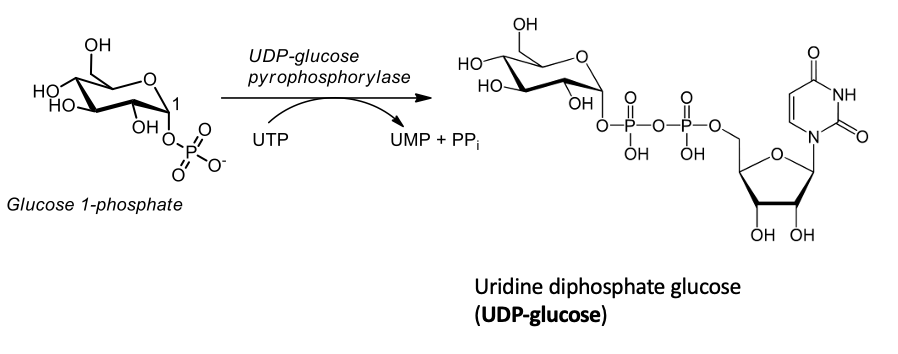
This α-D-glucose molecule is added to the growing glycogen chain in step 4
Step 3: synthesis of an initiating primer
Glycogenin is a priming glucosyltransferase
Glycogenin serves as an acceptor of glucose residues
A glucose unit is attached to glycogenin via Tyr –OH group
This forms the initiating primer
Incoming α-D-glucose units from UDP-glucose are transferred to the primer
Chain begins to grow
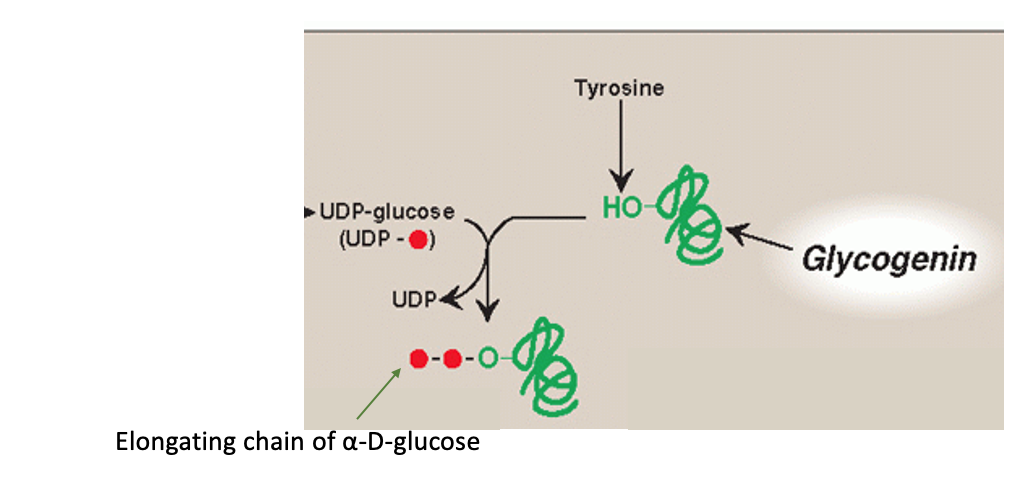
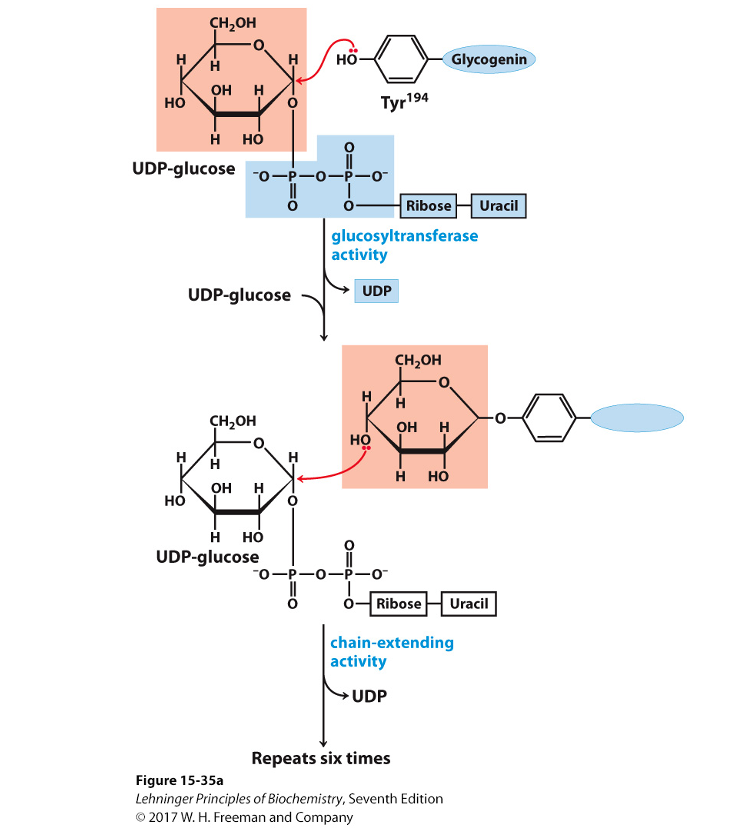
Step 4: Chain Elongation by Glycogen Synthase
The transfer of glucose units from UDP-glucose by glycogen synthase leads to chain elongation of glycogen
The transfer occurs at the non-reducing end
The reducing end is the glycogenin-linked region
The glucose units are linked serially as α-(1,4) glycosidic bonds
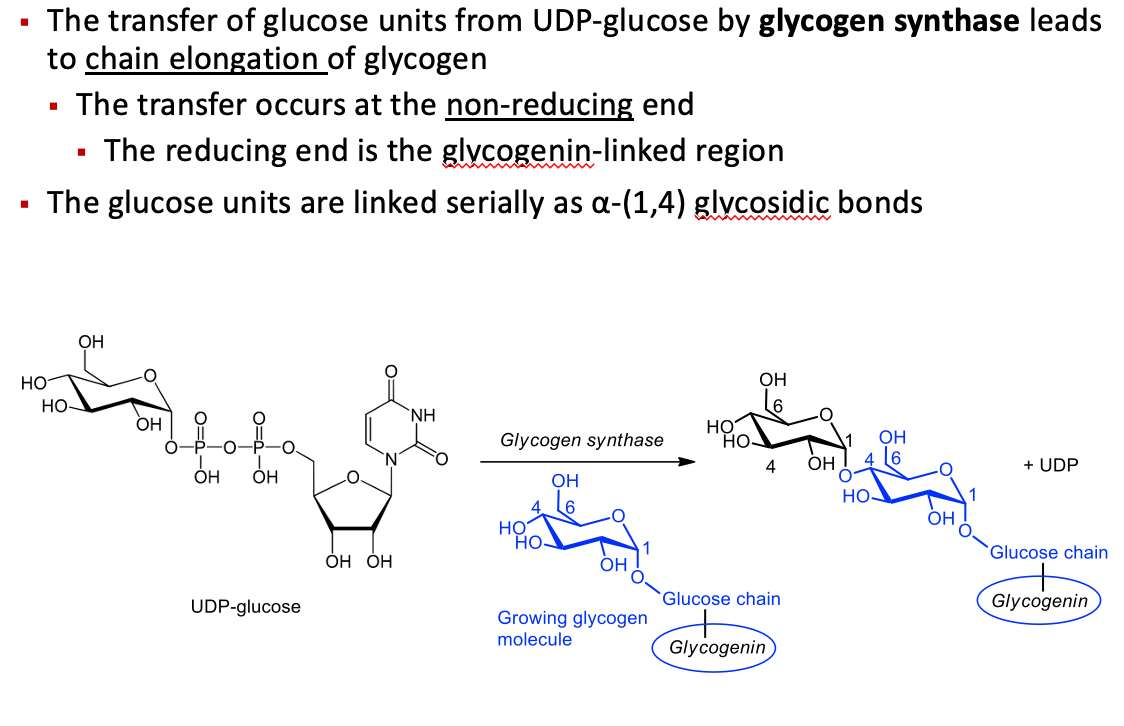
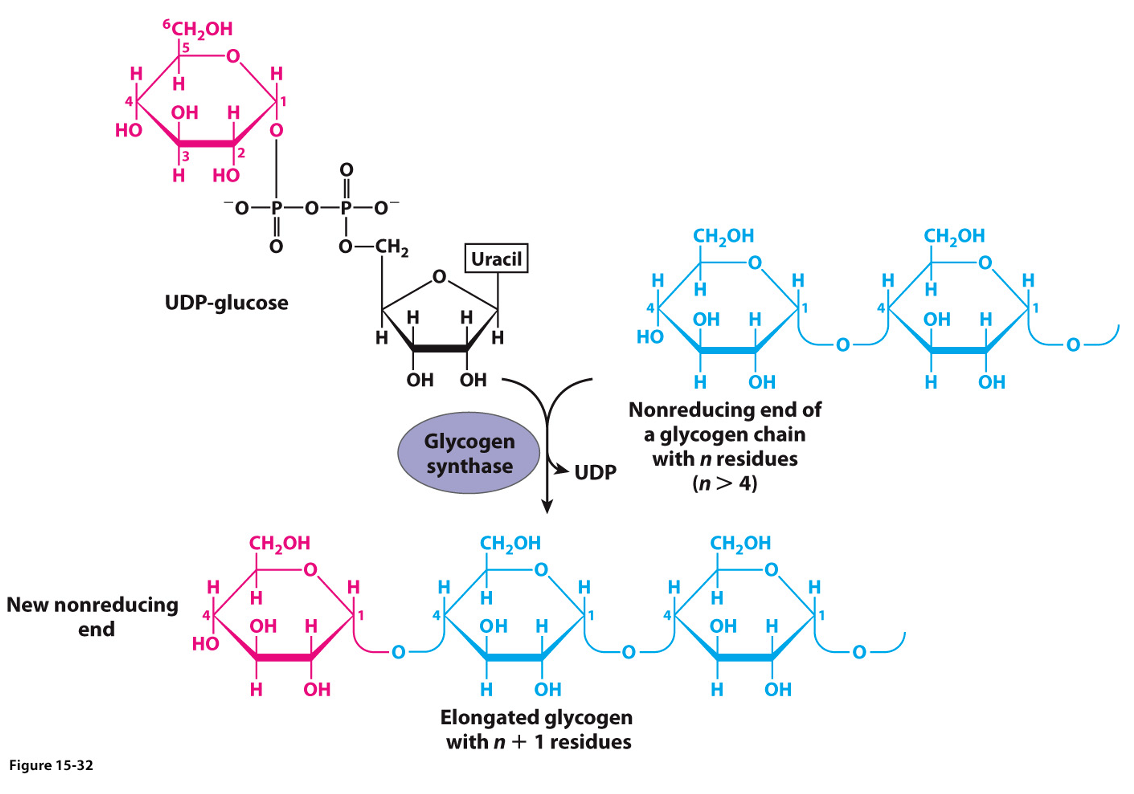
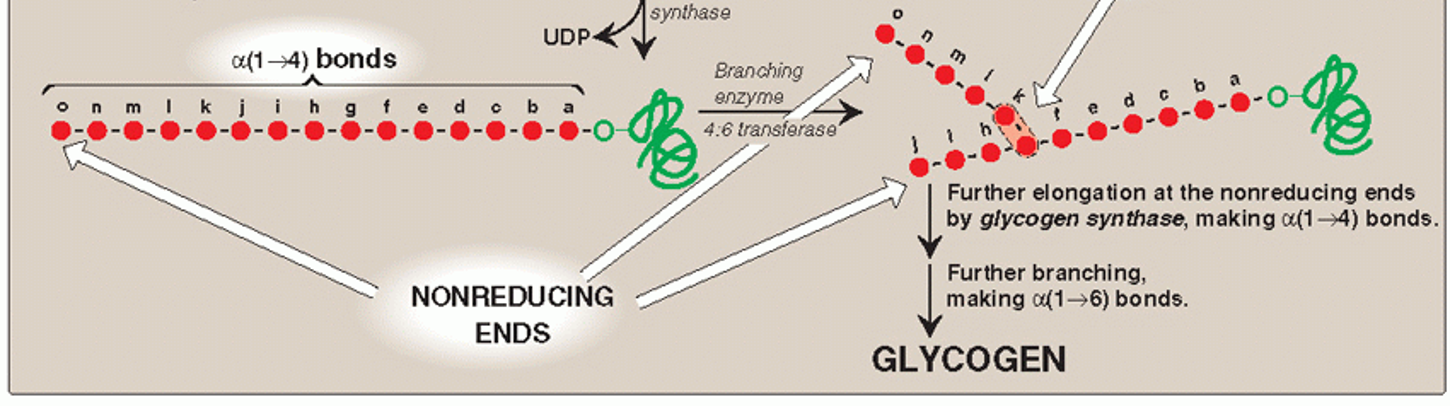
Step 5: branching
4:6 transferase (glycogen branching enzyme)
Amylo-α-(1,4),α-(1,6) transglucosidase
Transfers 5-8 residues from the non-reducing end [which has α-(1,4) linkage] to another glucose 1-phosphate residue on the chain
This creates a branch point with [α-(1,6) linkage] point
Branching allows more non-reducing ends to be created
Helps packs in more α-D-glucose units on the glycogen particle
Glycogenolysis
Glycogenolysis
Definition: The breakdown of glycogen to release glucose 1-phosphate and α-D-glucose
Glycogenolysis is a catabolic pathway (anabolic, catabolic)
A different set of enzymes than in glycogenesis
Pathway is not a direct reversal of the reactions of glycogenesis
Glycogenolysis involves the following steps, catalyzed by specific enzymes
step #
action type
enzyme
1
shortening of glycosidic chains
glycogen phosphorylase
2
de-branching (move 3 resides)
4:4 transferase (debranching enzyme #1)
3
de-branching (move 1 glucose)
1:6 glucosidase (debranching enzyme #2)
4
conversion of glucose 1-phosphate to glucose 6-phosphate
phosphoglucomutase
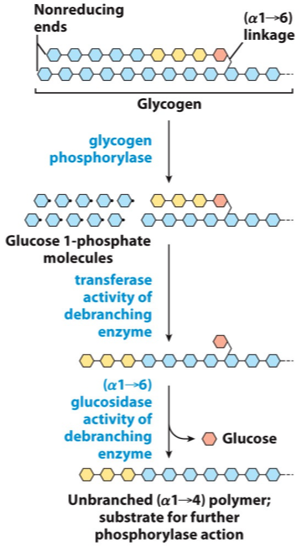
Step 1: shortening of glycosidic chains
Glycogen phosphorylase
Phosphorolysis
Pyridoxal phosphate (prosthetic group)
α-(1,4)-linkage of glycogen cleaved from non-reducing end
Glucose 1-phosphate is released
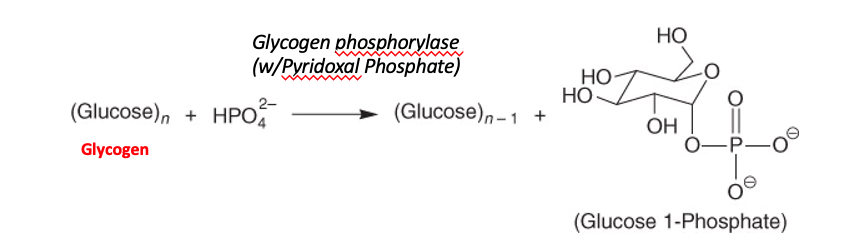
Enzyme continues until four glucosyl units remain from the branch point
This four unit “stump” is called limit dextrin
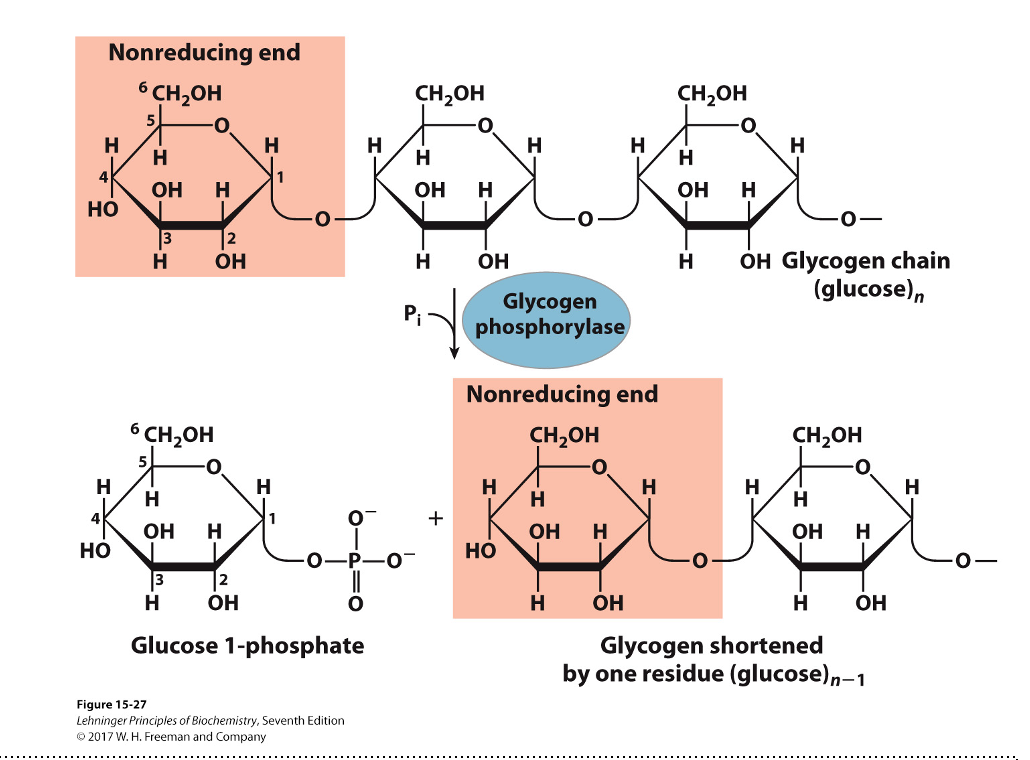
Step 2: de-branching (remove 3 resides)
Limit dextrin initiates the action of a debranching enzyme
4:4 transferase enzyme (debranching enzyme #1)
Oligo-α-(1,4), α-(1,4)-glucantransferase
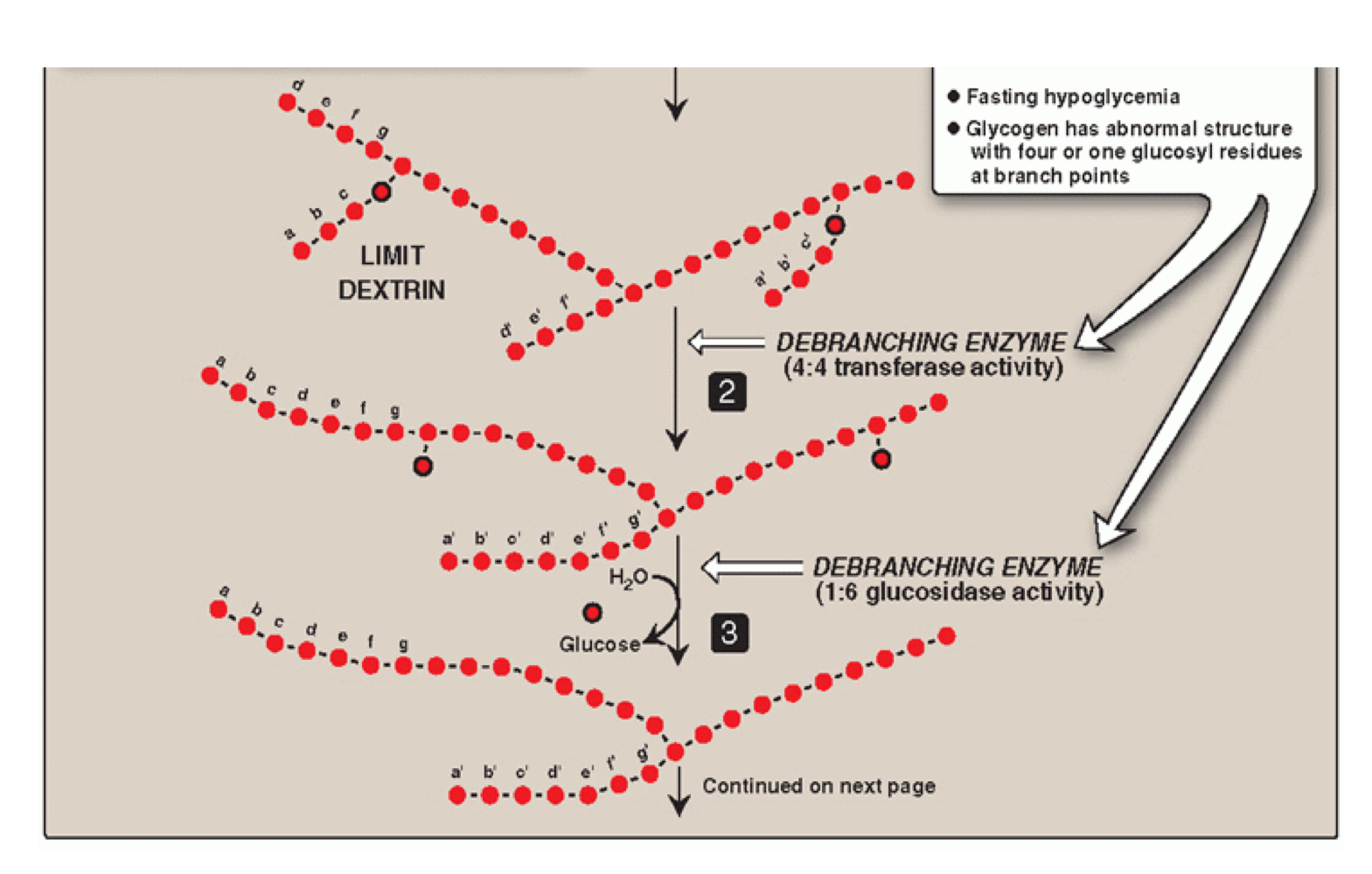
4:4 transferase enzyme removes 3 residues from limit dextrin
These 3 residues are transferred to the non-reducing end of another chain
1 residue is left attached (removed in step 3)
One α-(1,4) bond is broken and a new α-(1,4) bond is made
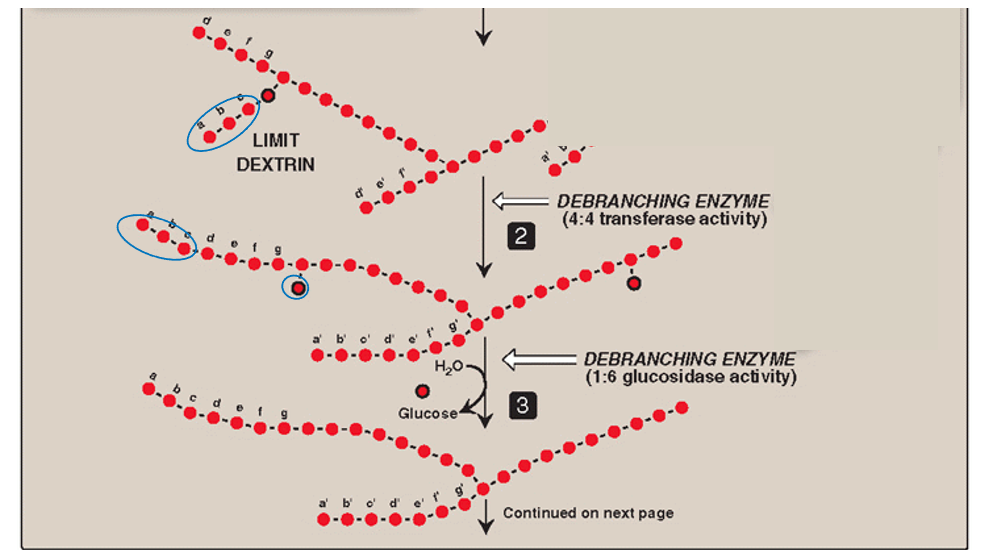
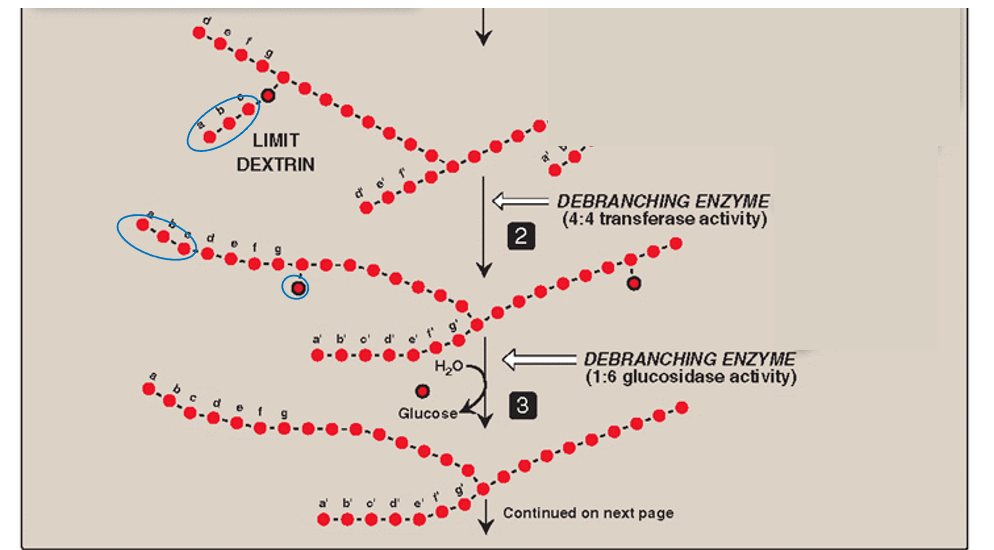
Step 3: de-branching (remove 1 glucose)
The lone glucose residue attached by α-(1,6)-glycosidic bond is removed by hydrolysis
Amylo-α-(1,6) glucosidase activity (1:6 glucosidase)
Debranching enzyme #2
Glycogen phosphorylase is used again for degradation if needed
Step 4: Conversion of glucose 1-phosphate to glucose 6-phosphate
Glucose 1-phosphate (from Step 1) is converted to glucose 6-phosphate by phosphoglucomutase
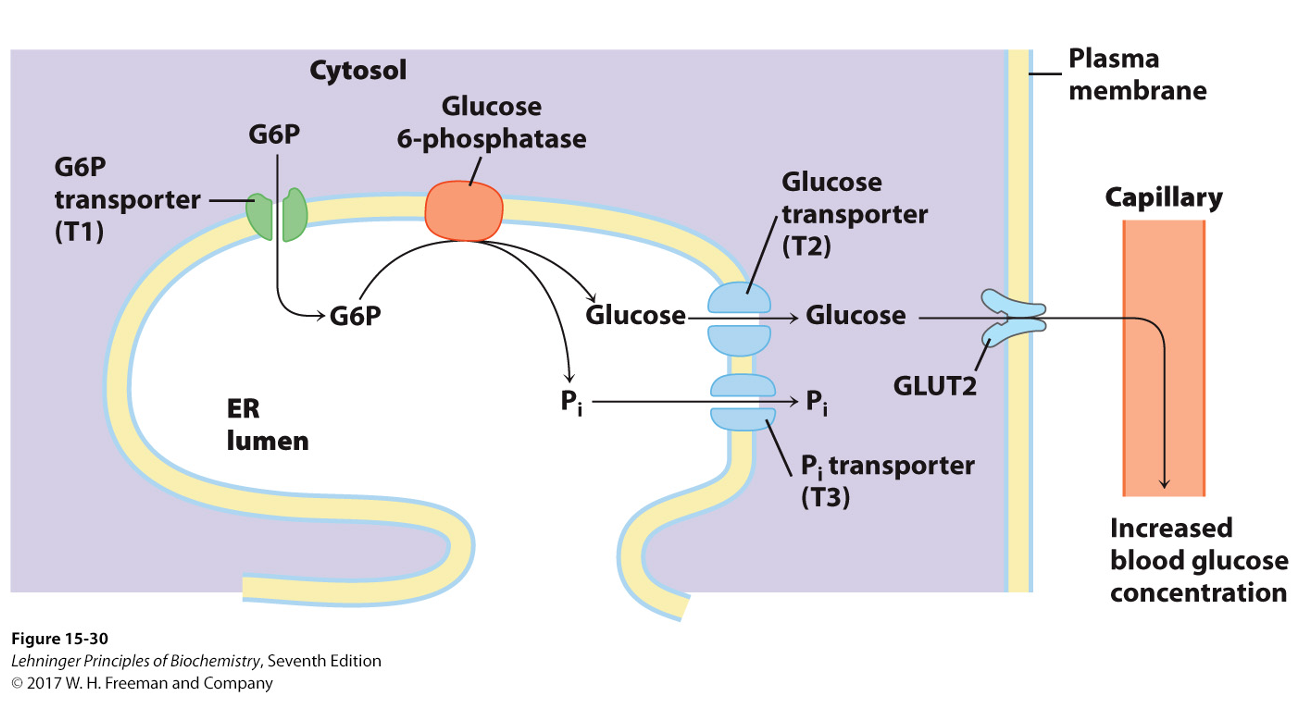
In the Liver
Glucose 6-phosphate is transported to the ER by glucose 6-phosphate
translocase
In the ER, glucose 6-phosphate is converted to glucose by glucose 6- phosphatase
Glucose is released into the blood stream
In the Muscle
Glucose 6-phosphate levels build up
Muscle lacks glucose 6-phosphatase (see gluconeogenesis)
What should the muscle cells do now? They will not make glucose … ☹
Cell needs energy. What should it do? Start GLYCOLYSIS ... from step 2 (Glucose 6-phosphate to Fructose 6-phosphate)
Glycogen Breakdown: Summary
Glycogen phosphorylase works on non-reducing ends until it reaches four residues from an (α1 → 6) branch point.
Debranching enzyme #1 transfers a block of three residues to the non-reducing end of the chain
Debranching enzyme #2 cleaves the single remaining (α1 → 6)-linked glucose, which becomes a free glucose unit (i.e., NOT glucose-1- phosphate).
Regulation of Glycogen Metabolism
Synthesis and breakdown of glycogen are tightly regulated
Importance in maintaining blood glucose levels
In the liver
Well fed state: glycogenesis accelerates
Fasting state: glycogenolysis accelerates
In the muscle
Active exercise: glycogenolysis accelerates
At rest: glycogenesis accelerates
Regulation occurs at two levels
Receptor level
Allosteric control of glycogenesis and glycogenolysis
Hormonal level
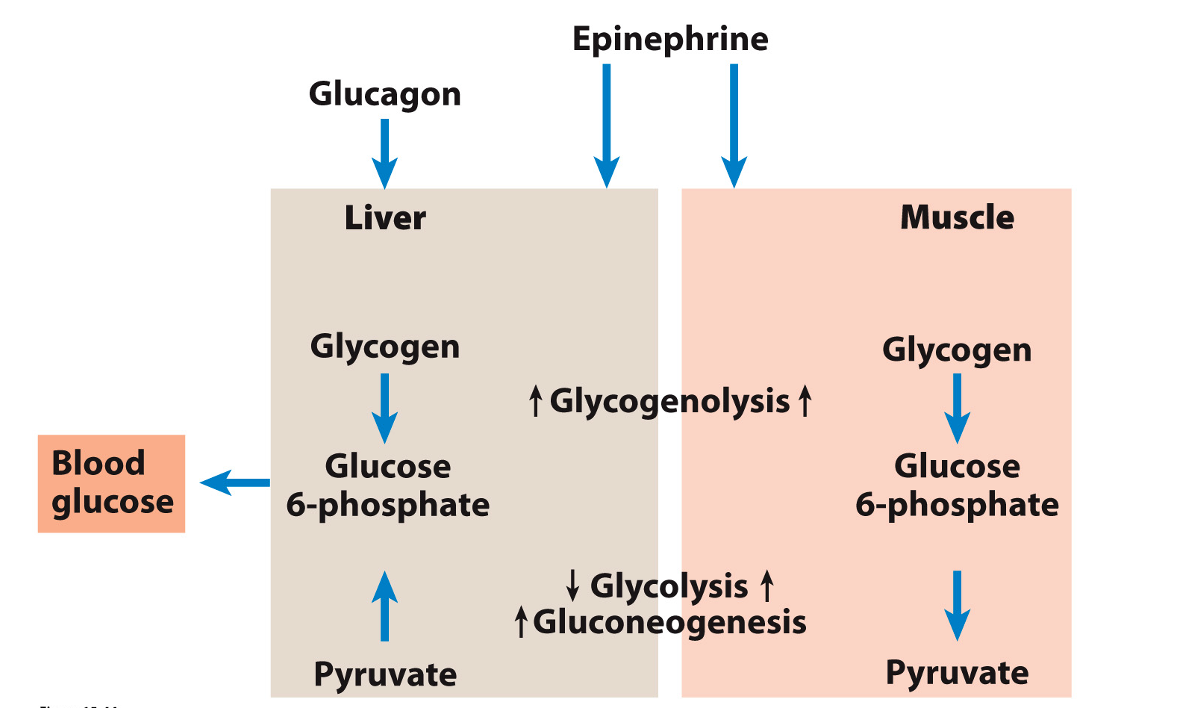
Glycogen phosphorylase and glycogen synthase are hormonally regulated by insulin, glucagon and epinephrine (aka adrenaline)
Insulin generally opposes the effects of glucagon and epinephrine
Insulin favors glycogen synthesis
Glucagon and epinephrine favor glycogen breakdown
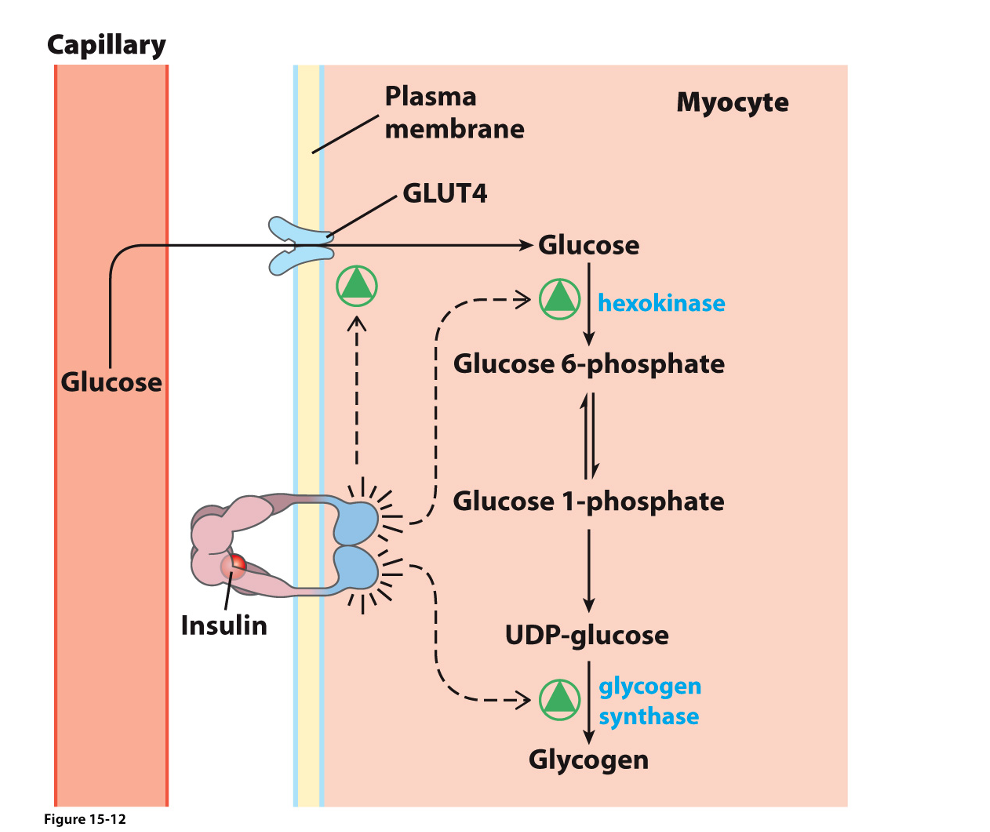
Hormonal Stimulation of Glycogen Synthesis
Glycogen synthase a : active form, dephosphorylated
Glycogen synthase b : inactive form, phosphorylated
Glycogen phosphorylase a : active form, phosphorylated
Glycogen phosphorylase b : inactive form, dephosphorylated
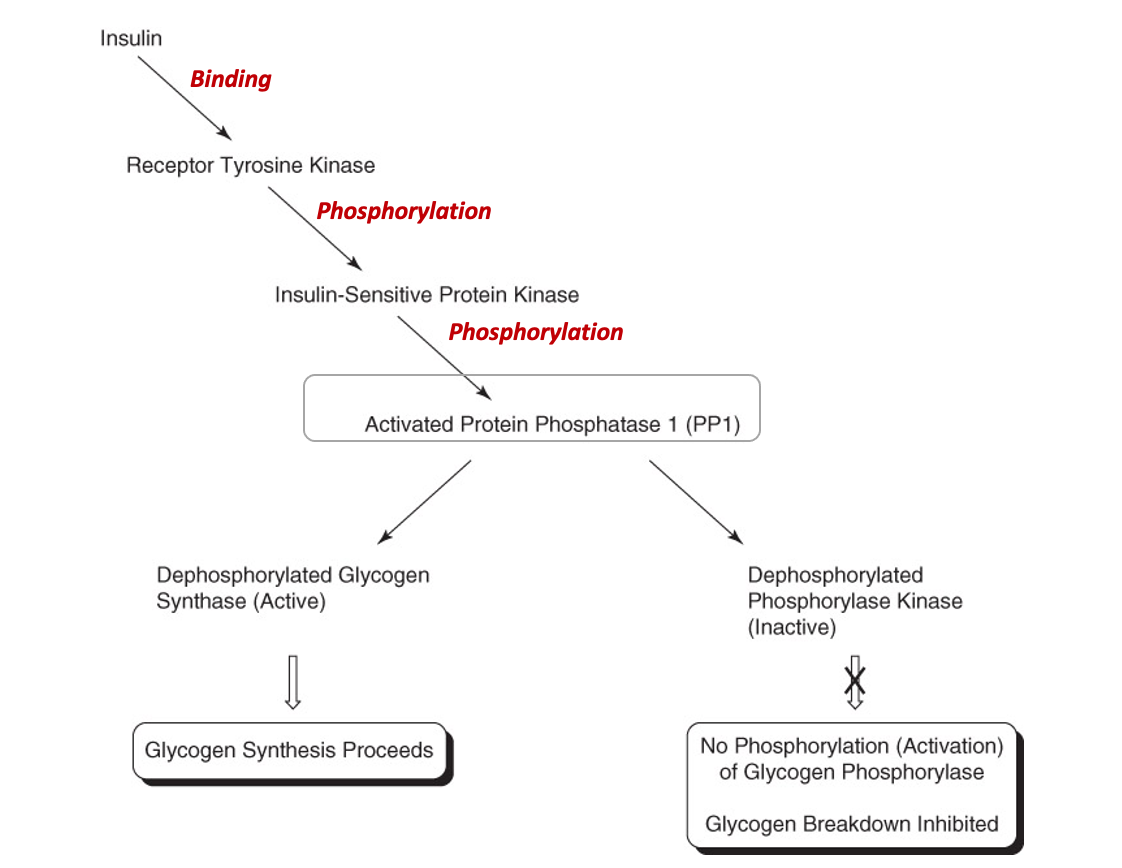
Insulin binds to a receptor and activates a tyrosine kinase
The receptor tyrosine kinase phosphorylates an insulin-sensitive kinase
Insulin-sensitive kinase phosphorylates Phosphatase-1
“active phosphate group” is created
Activated Protein Phosphatase-1 (PP-1) activates glycogen synthase
Leads to glycogen synthesis
Activated Protein Phosphatase-1 (PP-1) inhibits phosphorylase kinase
Phosphorylase kinase activates glycogen phosphorylase
Inhibition of phosphorylase kinase thus prevents the breakdown of glycogen
Indirect inhibition of glycogen phosphorylase
Influence of insulin on glucose transport, glycolysis, and glycogenesis
Hormonal Stimulation of Glycogen Breakdown
Glucagon and epinephrine promotes glycogen breakdown and stops glycogen synthesis
Glycogen synthase exists in two forms:
Glycogen synthase a : active form, dephosphorylated
Glycogen synthase b : inactive form, phosphorylated
Glycogen phosphorylase exists in two forms:
Glycogen phosphorylase a : active form, phosphorylated
Glycogen phosphorylase b : inactive form, dephosphorylated
Hormonal Regulation of Glycogen Breakdown
Glycogen synthase a : active form, dephosphorylated
Glycogen synthase b : inactive form, phosphorylated
Glycogen phosphorylase a : active form, phosphorylated
Glycogen phosphorylase b : inactive form, dephosphorylated
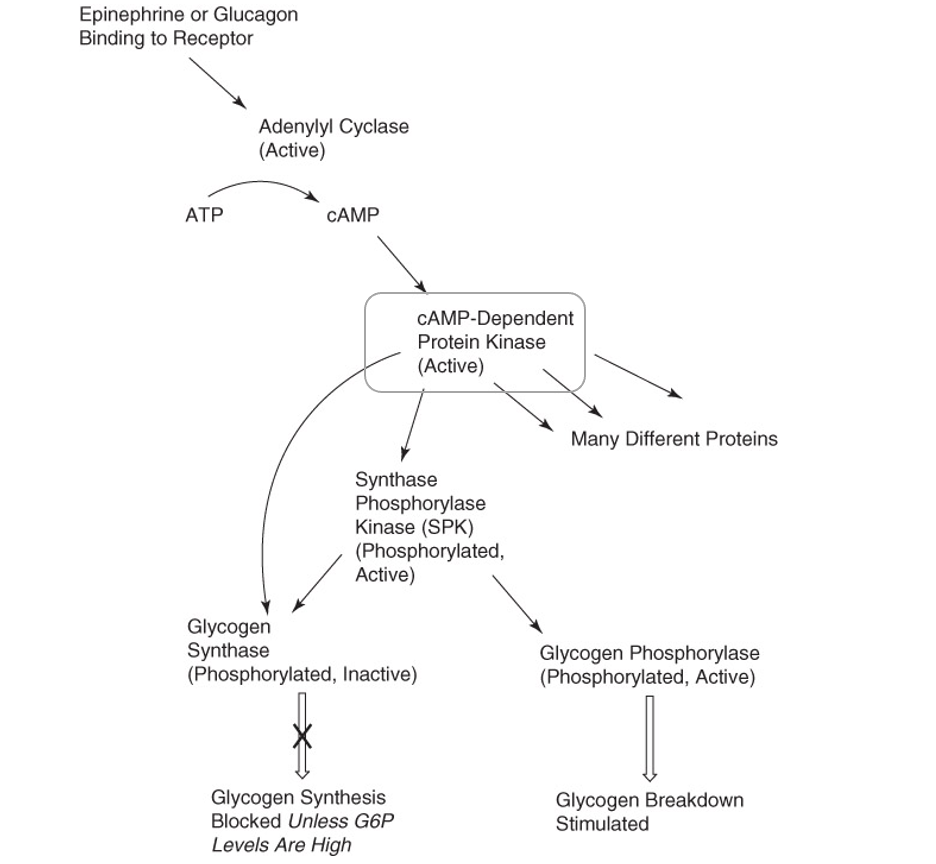
Epinephrine or glucagon activates adenylyl cyclase
Adenylyl cyclase catalyzes the generation of cyclic AMP (cAMP)
cAMP activates a cAMP-dependent protein kinase
Higher the levels of cAMP, greater the number of activated protein kinase molecules
cAMP-dependent protein kinase phosphorylates both glycogen synthase and glycogen phosphorylase
Glycogen synthase becomes inactive; glycogen synthesis stops
Glycogen phosphorylase becomes active; glycogen breakdown is favored
Influence of glucagon and epinephrine on glycolysis, gluconeogenesis and glycogenesis
Coordination of glycogen breakdown with glycogen synthesis
Glycogen phosphorylase
Glycogen synthase
Synthesis and breakdown of glycogen is coordinated
When one process is active, the other is shut off
The hormone signaling system is common to both pathways
The effects of phosphorylation on the two enzymes have opposing effects
Reciprocal regulation!
Flow Diagram of Glycogen Metabolism