Chapter 15 - Fecal Analysis
Physiology
- Many species of bacteria make up the normal flora of the intestines, and they produce the strong odor associated with feces and intestinal gas (flatus).
- Carbohydrates resistant to digestion and lactose (for those with intolerance) passes through the upper intestine and are metabolized by bacteria in the lower intestine, producing large amounts of flatus.
- Although digestion of ingested proteins, carbohydrates, and fats takes place throughout the alimentary tract, the small intestine is the primary site for the final breakdown and reabsorption of these compounds.
- Digestive enzymes secreted into the small intestine by the include trypsin, chymotrypsin, amino peptidase and lipase by the pancreas, and bile salts by the liver.
- If undigested or unreabsorbed material appear in the feces, the patient may be exhibiting symptoms of maldigestion and malabsorption.
- Water and electrolytes are readily absorbed in both the small and large intestines, resulting in a fecal electrolyte content that is similar to that of plasma.
- The large intestine is capable of absorbing approximately 3000 mL of water.
- When the amount of water reaching the large intestine exceeds this amount, it is excreted with the solid fecal material, causing diarrhea.
- When the amount is below this amount, water is reabsorbed from the feces and produces small hard stools, causing constipation.
Diarrhea
Diarrhea is defined as an increase in daily stool weight above 200 g with increased liquidity and frequency of more than three times per day.
Diarrhea classification can be based on four factors: duration of the illness, mechanism, severity, and stool characteristics.
Diarrhea lasting less than 4 weeks is defined as acute, and diarrhea persisting for more than 4 weeks is termed chronic diarrhea.
The major mechanisms of diarrhea are secretory and osmotic.
The laboratory tests used to differentiate these mechanisms are fecal electrolytes (fecal sodium, fecal potassium), fecal osmolality, and stool pH.
- The total fecal osmolarity is close to the serum osmolality (290 mOsm/kg).
- The fecal sodium and fecal potassium results are used to calculate the fecal osmotic gap, and it is calculated as follows:
Osmotic gap = 290 – [2 (fecal sodium + fecal potassium)]
| Secretory Diarrhea | Osmotic Diarrhea | |
|---|---|---|
| Causative Agent | bacterial, viral and protozoan infections, drugs, hormones, inflammatory bowel disease, endocrine disorders, neoplasms and collagen vascular disease | disaccharidase deficiency, malabsorption, laxatives, magnesium-containing antacids, amebiasis and antibiotic administration |
| Osmotic Gap | <50 mOsm/kg | >50 mOsm/kg |
| Electrolytes | increased electrolytes | negligible electrolytes |
| Fecal Fluid pH | >5.6 | <5.6 |
| Diagnostic Tests | stool cultures, ova and parasite examinations, rotavirus immunoassay, fecal leukocytes | fecal fat examination, muscle fiber detection, trypsin screening, Clinitest, D-xylose tolerance test, lactose tolerance test, fecal electrolytes, stool pH, fecal osmolality |
- Rapid gastric emptying (RGE) dumping syndrome describes hypermotility of the stomach and the shortened gastric emptying half-time, which causes the small intestine to fill too quickly with undigested food from the stomach.
- Healthy individuals have a gastric emptying half-time range of 35 to 100 minutes, which varies with age and gender; in this case, the time is less than 35 minutes.
- The main causes of dumping syndrome include gastrectomy, gastric bypass surgery, postvagotomy status, Zollinger-Ellison syndrome, duodenal ulcer disease and diabetes mellitus.
- Alterations in the motor functions of the stomach result in the accumulation of large amounts of osmotically active solids and liquids to be transported into the small intestine.
- Approximately 10% of patients get RGE after gastric surgery because of alterations in the fundic tone, duodenal feedback and GI hormones which control gastric emptying.
- Hypoglycemia is often a complication of dumping syndrome.
- RGE can be divided into early dumping and late dumping depending upon how soon after a meal the symptoms occur.
| Early Dumping | Late Dumping |
|---|---|
| occurs 10 to 30 minutes after eating | occurs 2 to 3 hours after eating |
| symptoms of nausea, vomiting, bloating, cramping, diarrhea, dizziness and fatigue | symptoms of weakness, sweating and dizziness |
Steatorrhea
- It is an an increase in stool fat (>6 g per day) caused by the absence of bile salts that assist pancreatic lipase in the breakdown and subsequent reabsorption of triglycerides.
- Steatorrhea may be present in both maldigestion and malabsorption conditions and can be distinguished by the D-xylose test.
- D-xylose is a sugar that does not need to be digested but does need to be absorbed to be present in the urine.
- If urine D-xylose is low, the resulting steatorrhea would indicate a malabsorption condition.
- A normal D-xylose test indicates pancreatitis.
Specimen Collection
- Patients should be instructed to collect the specimen in a clean container, such as a bedpan or disposable container, and transfer it to the laboratory container.
- The specimen must not be contaminated with urine or toilet water, which may contain chemical disinfectants.
- Containers that contain preservatives for ova and parasites must not be used to collect specimens intended for other tests.
- Random specimens suitable for qualitative testing are usually collected in plastic or glass containers with screw-capped tops similar to those used for urine specimens.
- For quantitative testing, timed specimens are required.
- Because of the variability of bowel habits and the transit time required for food to pass through the digestive tract, the most representative sample is a 3-day collection.
- These specimens are frequently collected in paint cans to accommodate the specimen quantity and facilitate emulsification prior to testing.
- Care must be taken when opening any fecal specimen to slowly release gas that has accumulated within the container.
- Patients must be cautioned not to contaminate the outside of the container.
Macroscopic Screening
Color
| Color | Clinical Significance |
|---|---|
| brown | normal (intestinal oxidation of stercobilinogen to urobilin) |
| pale yellow, white, gray | blockage of the bile duct, barium sulfate |
| red | blood from the lower gastrointestinal tract, rifampin, beets, food coloring |
| black, tarry | blood from the esophagus, stomach or duodenum (it takes approximately 3 days to appear in the stool; during this time, hemoglobin is degraded), iron, charcoal, bismuth (antacids) |
| green | oral antibiotics (oxidation of fecal bilirubin to biliverdin), green vegetables or food coloring |
Appearance
| Appearance | Clinical Significance |
|---|---|
| watery | diarrhea |
| small, hard | constipation |
| slender, ribbon-like | obstruction in the intestines |
| bulky, frothy, greasy, foul odor, may float | biliary obstruction, steatorrhea |
| mucus-coated | intestinal inflammation (pathologic colitis), irritation (excessive straining during excretion) |
| if blood-streaked, damage to the intestinal walls, possibly caused by bacterial or amebic dysentery or malignancy |
Microscopic Screening
Fecal Leukocytes
Leukocytes, primarily neutrophils, are seen if the intestinal mucosa is affected.
- Staphylococcus aureus and Vibrio spp., viruses, and parasites usually do not cause the appearance of fecal leukocytes.
- In an examination of preparations under high power, as few as three neutrophils per high-power field can be indicative of an invasive condition.
- Using oil immersion, the finding of any neutrophils has approximately 70% sensitivity for the presence of invasive bacteria.
- A lactoferrin latex agglutination test is available for the detection of fecal leukocytes and remains sensitive in refrigerated and frozen specimens.
- The presence of lactoferrin, a component of granulocyte secondary granules, is indicative of an invasive bacterial pathogen.
Specimens can be examined as wet preparations stained with methylene blue or as dried smears stained with Wright’s or Gram stain.
- Methylene blue staining is the faster procedure but may be more difficult to interpret.
- Dried preparations stained with either Wright’s or Gram stains provide permanent slides for evaluation.
- An additional advantage of the Gram stain is the observation of Gram-positive and Gram-negative bacteria, which could aid in the initial treatment.
- To perform methylene blue staining:
- Place mucus or a drop of liquid stool on a slide.
- Add two drops Löffler methylene blue and mix with a wooden applicator stick.
- Allow to stand 2–3 minutes.
- Examine for neutrophils under high power.
Muscle Fibers
It can be helpful in the diagnosis and monitoring of patients with pancreatic insufficiency.
- Increased striated fibers may also be seen in biliary obstruction and gastrocolic fistulas.
It is frequently ordered in conjunction with microscopic examinations for fecal fats.
Slides for muscle fiber detection are prepared by emulsifying a small amount of stool in 10% alcoholic eosin, which enhances the muscle fiber striations.
- The entire slide is examined for exactly 5 minutes, and the number of red-stained fibers with well-preserved striations is counted.
- Only undigested fibers are counted, and the presence of more than 10 is reported as increased.
- Undigested fibers have visible striations running both vertically and horizontally.
- Partially digested fibers exhibit striations in only one direction.
- Digested fibers have no visible striations.
- To perform muscle fiber examination:
- Emulsify a small amount of stool in two drops of 10% eosin in alcohol.
- Add the coverslip and let it stand 3 minutes.
- Examine under high power and count the number of undigested fibers.
To produce a representative sample, patients should be instructed to include red meat in their diet prior to collecting the specimen.
Specimens should be examined within 24 hours of collection.
Qualitative Fecal Fats
Specimens from suspected cases of steatorrhea can be screened microscopically for the presence of excess fecal fat.
The procedure can also be used to monitor patients undergoing treatment for malabsorption disorders.
In general, correlation between the qualitative and quantitative fecal fat procedures is good; however, additional unstained phospholipids and cholesterol esters are measured by the quantitative procedure.
Lipids included in the microscopic examination of feces are neutral fats (triglycerides), fatty acid salts (soaps), fatty acids, and cholesterol.
Neutral fats are readily stained by Sudan III and appear as large orange-red droplets, often located near the edge of the coverslip.
Observation of more than 60 droplets/highpower field can be indicative of steatorrhea; however, the split fat stain representing total fat content can provide a better indication.
The breakdown of neutral fats by bacterial lipase and the spontaneous hydrolysis of neutral fats may lower the neutral fat count.
This also precludes the comparison of the two slide tests to determine whether maldigestion or malabsorption is causing steatorrhea.
To perform neutral fat staining:
- Homogenize one part stool with two parts water.
- Mix emulsified stool with one drop 95% ethyl alcohol and two drops saturated Sudan III in 95% ethanol on a slide.
- Add a coverslip.
- Examine under high power and count the orange droplets per high-power field.
Soaps and fatty acids do not stain directly with Sudan III; therefore, a second slide must be examined after the specimen has been mixed with acetic acid and heated.
Examination of this slide reveals stained droplets that represent not only the free fatty acids but also the fatty acids produced by hydrolysis of the soaps and the neutral fats.
In an examination of this split fat slide, both the number and size of the fat droplets must be considered.
Normal specimens may contain as many as 100 small droplets, less than 4 μm in size, per high-power field.
- The same number of droplets measuring 1 to 8 μm is considered slightly increased, and 100 droplets measuring 6 to 75 μm is increased.
To perform split fat staining:
- Homogenize one part stool with two parts water.
- Mix emulsified stool with one drop of 36% acetic acid and two drops saturated Sudan III on a slide.
- Add a coverslip.
- Heat gently almost to boiling.
- Examine under high power, and count and measure the orange droplets per high-power field.
Cholesterol is stained by Sudan III after heating and as the specimen cools forms crystals that can be identified microscopically.
Chemical Testing
Occult Blood
Any bleeding in excess of 2.5 mL/150 g of stool is considered pathologically significant but is not visible, so fecal occult blood testing (FOBT) is necessary.
Annual testing for occult blood has a high positive predictive value for detection of colorectal cancer in the early stages and is recommended for persons older than age 50.
The most frequently encountered screening tests for occult blood are based on detection of the pseudoperoxidase activity of hemoglobin, reacting with hydrogen peroxide to oxidize a colorless compound to a colored compound:
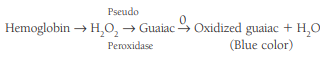
- The least sensitive reagent, guaiac, is preferred for routine testing.
- A normal stool can contain up to 2.5 mL of blood, so a less sensitive chemical reactant is more desirable.
- Pseudoperoxidase activity is present from hemoglobin and myoglobin in ingested meat and fish, certain vegetables and fruits, and some intestinal bacteria, so the sensitivity of the test must be decreased.
The immunochemical fecal occult blood test (iFOBT), is specific for the globin portion of human hemoglobin and uses anti-human hemoglobin antibodies, so it does not require dietary or drug restrictions.
- Hemoglobin from upper GI bleeding is degraded by bacterial and digestive enzymes before reaching the large intestine and is immunochemically nonreactive.
- It is more sensitive to lower GI bleeding that could be an indicator of colon cancer or other gastrointestinal disease and can be used for patients who are taking aspirin and other anti-inflammatory medications.
- It does not detect bleeding from other sources such as a bleeding ulcer, decreasing the chance for false positives.
Quantitative Fecal Fat Testing
It is used as a confirmatory test for steatorrhea.
It requires the collection of at least a 3-day specimen and a regulated intake of fat (100 g/d) in the diet prior to and during the collection period.
- Paint cans make excellent collection containers because the specimen must be homogenized prior to analysis, and this can be accomplished by placing the container on a conventional paint-can shaker.
- Refrigerating the specimen prevents any bacterial degradation.
The method routinely used for fecal fat measurement is the Van de Kamer titration, although gravimetric methods are available.
- Fecal lipids are converted to fatty acids and titrated to a neutral endpoint with sodium hydroxide.
- The fat content is reported as grams of fat or the coefficient of fat retention per 24 hours.
- Normal values based on a 100 g/d intake are 1 to 6 g/d or a coefficient of fat retention of at least 95%.
- The coefficient of fat retention is calculated as follows:
Another test, the acid steatocrit, is a more rapid and more convenient test to estimate the amount of fat excretion.
- The acid steatocrit is a reliable tool to monitor a patient’s response to therapy and screen for steatorrhea in pediatric populations.
- To perform this procedure:
- 0.5 g of feces from a spot collection is diluted 1 to 4 with deioinized water and vortexed for 2 minutes to homogenize the specimen.
- A volume of 5 N percholoric acid equal to 20% of the homogenate volume is added and the mixture is then vortexed for 30 seconds.
- Once the pH is <1, place the acid-homogenate mixture in 75 microliter plain hematocrit capillary tube and seal the end with wax.
- The capillary tube is centrifuged horizontally at 13,000 rpm for 15 minutes in a microhematocrit centrifuge.
- This separates fat as an upper layer overlying a solid fecal layer.
- The length of the fat and solid layers are measured using a magnifying lens.
- Calculate the acid steatocrit in percent.
- Calculate the fecal fat in grams per 24 hours.
- acid steatocrit in percent = (fatty layer length in cm) / [(fatty layer length in cm) + (solid layer length)] x 100
- fecal fat for adults in grams per 24 hours = [0.45 x (acid steatocrit in percent as a whole number)] – 0.43
- An acid steatocrit value
- fecal fat for children up to the age of 15 years in grams per 24 hours = [0.1939 x (acid steatocrit in percent as a whole number)] – 0 .2174
- Acid steatocrit is higher in infants and droppped with age.
- An acid steatocrit of <10% is indicative of steatorrhea in children.
Near-infrared reflectance spectroscopy (NIRA) is a rapid procedure for fecal fat that requires less stool handling by laboratory personnel.
- The test requires a 48-72 hour stool collection to exclude day-to-day variability, but it does not require reagents after homogenization of the sample.
- The result is based on the measurement and computed processing of signal data from reflectance of fecal surface, which is scanned with infrared light between 1400 nM and 2600 nM wavelength.
- The results are calculated from calibration derived from known samples.
- The technique quantitates water, fat and nitrogen in grams per 24 hours.
APT Test (Fetal Hemoglobin)
It distinguishes between fetal hemoglobin from hemoglobin A, and between maternal hemoglobins AS, CS, and SS, and fetal hemoglobin.
The material to be tested is emulsified in water to release hemoglobin (Hb) and, after centrifugation, 1% sodium hydroxide is added to the pink hemoglobincontaining supernatant.
- In the presence of alkali-resistant fetal hemoglobin, the solution remains pink (Hb F), whereas denaturation of the maternal hemoglobin (Hb A) produces a yellow-brown supernatant after standing for 2 minutes.
- To perform this procedure:
- Emulsify specimen in water and centrifuge.
- Divide pink supernatant into two tubes.
- Add 1% sodium hydroxide to one tube and wait 2 minutes.
- Compare color with that in the control tube.
- Prepare controls using cord blood and adult blood.
The presence of maternal thalassemia major would produce erroneous results owing to the high concentration of hemoglobin F.
Stool specimens should be tested when fresh.
- They may appear bloody but should not be black and tarry, because this would indicate already denatured hemoglobin.
Fecal Enzymes
- Analysis of the feces focuses primarily on the proteolytic enzymes trypsin, chymotrypsin, and elastase I.
- Historically, absence of trypsin has been screened for by exposing x-ray paper to stool emulsified in water.
- When trypsin is present in the stool, it digests the gelatin on the paper, leaving a clear area; however, the gelatin test is an insensitive procedure that detects only severe cases of pancreatic insufficiency.
- False-negative results may occur as the result of intestinal degradation of trypsin and the possible presence of trypsin inhibitors in the feces.
- The proteolytic activity of bacteria enzymes may produce false-positive results in old specimens.
- Fecal chymotrypsin is more resistant to intestinal degradation and is a more sensitive indicator of less severe cases of pancreatic insufficiency.
- It remains stable in fecal specimens for up to 10 days at room temperature.
- Chymotrypsin is capable of gelatin hydrolysis but is most frequently measured by spectrophotometric methods.
- Elastase I is present in high concentrations in pancreatic secretions and is strongly resistant to degradation.
- It accounts for about 6% of all secreted pancreated enzymes.
- Fecal elastase I is pancreas specific and its concentration is about five times higher than in pancreatic juice.
- It is not affected by motility disorders or mucosal defects.
- Elastase I can be measured by immunoassay using the ELISA kit and provides a very sensitive indicator of exocrine pancreatic insufficiency.
- It is easy to perform and requires only a single stool sample.
- The ELISA test uses monoclonal antibodies against human pancreatic elastase-1; therefore, the result is specific for human enzyme and not affected by pancreatic enzyme replacement therapy.
- The test is specific in differentiating pancreatic from nonpancreatic causes in patients with steatorrhea.
Carbohydrates
- Carbohydrate malabsorption or intolerance (maldigestion) is primarily analyzed by serum and urine tests; however, an increased concentration of carbohydrate can be detected by performing a copper reduction test on the fecal specimen.
- The copper reduction test is performed using a Clinitest tablet and one part stool emulsified in two parts water.
- A result of 0.5 g/dL is considered indicative of carbohydrate intolerance.
- The Clinitest on stools can distinguish between diarrhea caused by abnormal excretion of reducing sugars and those caused by various viruses and parasites.
- In premature infants there is correlation between a positive Clinitest and inflammatory necrotizing entercolitis.
- Sucrose is not detected by the Clinitest method because it is not a reducing sugar. A positive result would be followed by more specific serum carbohydrate tolerance tests such as the D-xylose test and the lactose tolerance test.
- Stool chromatography to identify the malabsorbed carbohydrate is available but rarely necessary for the diagnosis of sugar intolerance.
- Small-bowel biopsy specimens for histologic examination and the assay of disaccharidase enzyme activity differentiate primary from secondary disaccharidase intolerance.
- Testing for fecal reducing substances detects congenital disaccharidase deficiencies as well as enzyme deficiencies due to nonspecific mucosal injury.
- Fecal carbohydrate testing is most valuable in assessing cases of infant diarrhea and may be accompanied by a pH determination.
- Normal stool pH is between 7 and 8; however, increased use of carbohydrates by intestinal bacterial fermentation increases the lactic acid level and lowers the pH to below 5.5 in cases of carbohydrate disorders.