Atomic structure and electron configuration
Differences in chemical reactivity between elements are based on the number and spatial distribution of their electrons
Each electron shell has a different energy level: the closer that it is to the nucleus, the more energy it has.
Shell capacity: 1n = 2, 2n = 8, 3n = 18.
the number of electrons in the valence shell determines its reactivity. atoms are most stable when their valence shell is full.
most elements important to biology are stable with 8 valence electrons, this is known as the octet rule.
In general, the number of valence electrons are the same in a row, and increases from left to right within a row
Subshells and orbitals
Subshells are designated by the letters s, p, d, and f, and each letter represents a different shape.
S: spherical orbital
P: 3 dumbbell shaped orbitals at right angles to each other.
most of organic chemistry involves interactions between s and p orbitals, but subshells d and f are more complex and involve 5 and 7 orbitals, respectively.
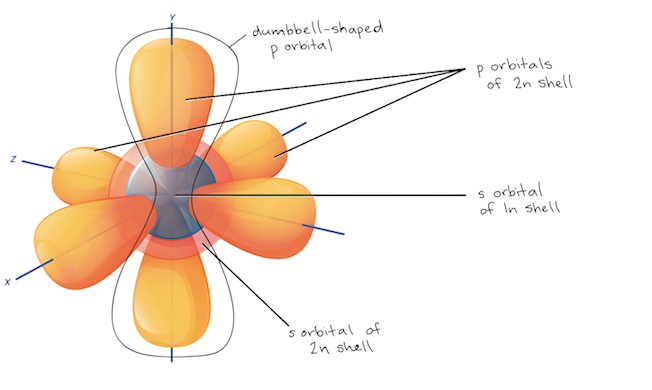
the first electron shell,1n, corresponds to the 1s orbital, which can be written as 1s^1 (for hydrogen).
Shell is the energy level, like 1 or 2, but subshells are your 1s, and 2p.
the S subshell can fit 2 electrons; the p, 6,; the d, 10; the f, 14 (or 2 per orbital)
Aufbau principle
meaning the building principle.
in electron configuration, this goes from 1s to 2s, 2p, 3s, 3p, 4s, 3d, 4p and 5s..
the first 2 columns of the periodic table is the S block (and helium), transition metals are d-block, and noble gases, nonmetals, and metalloids are p-block.