Chapter 16 Sense Organs - Flashcards (Question and Answer)
16.1 Properties and Types of Sensory Receptors
Receptor: a structure specialized to detect a stimulus
Some receptors are bare nerve endings (e.g., free nerve endings for pain and temperature).
Others are true sense organs: a structure that combines nerve tissue surrounded by other tissues that enhance response to a certain type of stimulus (e.g., the eye, ear, or a dendrite wrapped in connective tissue like a Pacinian corpuscle).
Accessory tissues may include epithelium, muscle, or connective tissue.
They range in size and complexity: from complex organs like the eye and ear to a simple dendrite wrapped in connective tissue.
Receptor transduction: conversion of one form of energy to another
Fundamental purpose: convert stimulus energy (e.g., light, heat, chemical, mechanical stress, sound) into electrochemical nerve signals (action potentials).
Two stages in the sensory process:
Sensation: the receptor detects the stimulus, which creates a receptor potential (a small local electrical change in the receptor cell's membrane potential, often a graded potential).
If the receptor potential is strong enough (reaching threshold), it triggers the firing of action potentials in the sensory neuron, which are then transmitted to the brain.
Perception: the conscious experience and interpretation of a stimulus by the cerebral cortex.
Not all sensations reach conscious perception; many signals are filtered out by lower brain centers (e.g., thalamus, brainstem reticular formation) before reaching the cerebral cortex, preventing sensory overload.
Information transmitted by receptors (4 kinds):
Modality: the type of stimulus or the perception produced (e.g., vision, hearing, taste, touch, pain, temperature). This is encoded by the "labeled line code," meaning that each nerve fiber to the brain is dedicated to a specific stimulus modality.
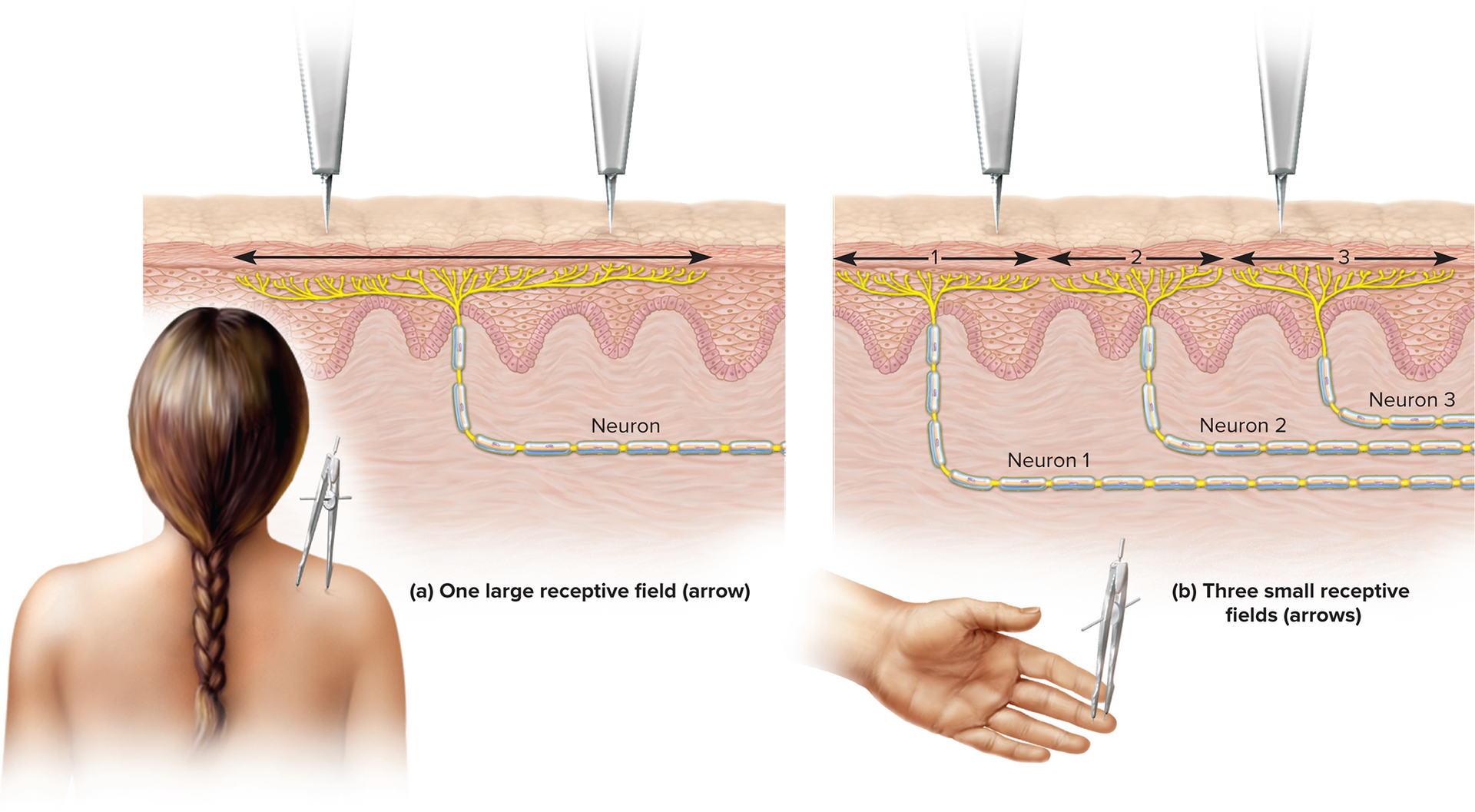
Location: where a stimulus is located or where it originates. This is determined by which nerve fibers are firing; the receptive field is the area within which a sensory neuron detects stimuli.
Intensity: the strength of the stimulus. This is encoded by the firing rate of neurons, the number of neurons activated, and which neurons respond.
Duration: how long the stimulus lasts. This is encoded by changes in the firing frequency of nerve fibers over time.
Modality encoding: determined by which specific brain region is wired to receive the information from a particular type of receptor. For instance, signals from photoreceptors go to the visual cortex, and signals from mechanoreceptors go to the somatosensory cortex.
Location encoding: determined by which specific nerve fibers are firing; the receptive field is the area within which a sensory neuron detects stimuli. The brain identifies the origin of the signal based on which neuron is sending it.
Receptive field size affects resolution; small receptive fields (e.g., on the fingertips, tongue) enable fine two-point discrimination and higher acuity. Large receptive fields (e.g., on the back) provide less precise localization.
Intensity encoding (three mechanisms):
Which fibers respond: Weak stimuli activate only the most sensitive neurons with low thresholds. Stronger stimuli recruit additional neurons with progressively higher thresholds, expanding the area of activation.
How many fibers respond: More intense stimuli activate a greater number of neurons within the receptive field, a process called spatial summation.
How fast fibers fire: The firing frequency (rate of action potentials per unit of time) increases as stimulus intensity rises. This is known as temporal summation.
Duration encoding: changes in firing frequency over time signify how long a stimulus persists.
Sensory adaptation: a phenomenon where, in response to a prolonged, unchanging stimulus, the firing rate of sensory neurons slows, leading to a reduced awareness or cessation of the sensation.
Phasic receptors: adapt quickly to a stimulus; they generate a burst of action potentials at the onset of the stimulus, then sharply reduce or stop signaling even if the stimulus continues (e.g., receptors for smell, hair movement, and cutaneous pressure).
Tonic receptors: adapt slowly; they continue to fire action potentials throughout the duration of the stimulus, providing sustained information (e.g., receptors for body position, muscle tension, and joint motion, which are critical for maintaining balance and posture).
Classification of receptors by stimulus modality
Thermoreceptors: detect changes in temperature; separate receptors exist for warm and cold stimuli.
Photoreceptors: specialized to detect light; primarily found in the eyes (retina).
Nociceptors: detect tissue injury or threatening damage; responsible for the sensation of pain. They respond to mechanical, thermal, or chemical stimuli that are damaging.
Chemoreceptors: respond to chemicals, including odors (olfaction), tastes (gustation), and changes in body fluid composition (e.g., blood pH, CO2 levels).
Mechanoreceptors: detect physical deformation of tissue caused by vibration, touch, pressure, stretch, or tension; this category includes the organs of hearing and balance, as well as receptors in the skin and muscles.
Classification by origin of stimuli
Exteroceptors: detect external stimuli originating from outside the body (e.g., vision, hearing, taste, smell, and cutaneous sensations like touch, heat, cold, and pain on the skin).
Interoceptors: detect internal stimuli originating from within the body (e.g., stretch receptors in the stomach, intestines, and bladder; receptors for blood pressure, visceral pain, and nausea).
Proprioceptors: sense body position and movements; located in muscles, tendons, and joint capsules, providing information about limb position and body orientation.
Classification by distribution
General (somatosensory/somesthetic) senses: widely distributed throughout the body in the skin, muscles, tendons, joints, and viscera; include touch, pressure, stretch, temperature, pain, blood pressure, and blood composition.
Receptors may be simple (e.g., a bare dendrite) or complex (e.g., an encapsulated nerve ending).
Special senses: limited to the head; innervated by cranial nerves; involve complex sense organs (e.g., vision, hearing, equilibrium, taste, smell).
16.2 The General Senses
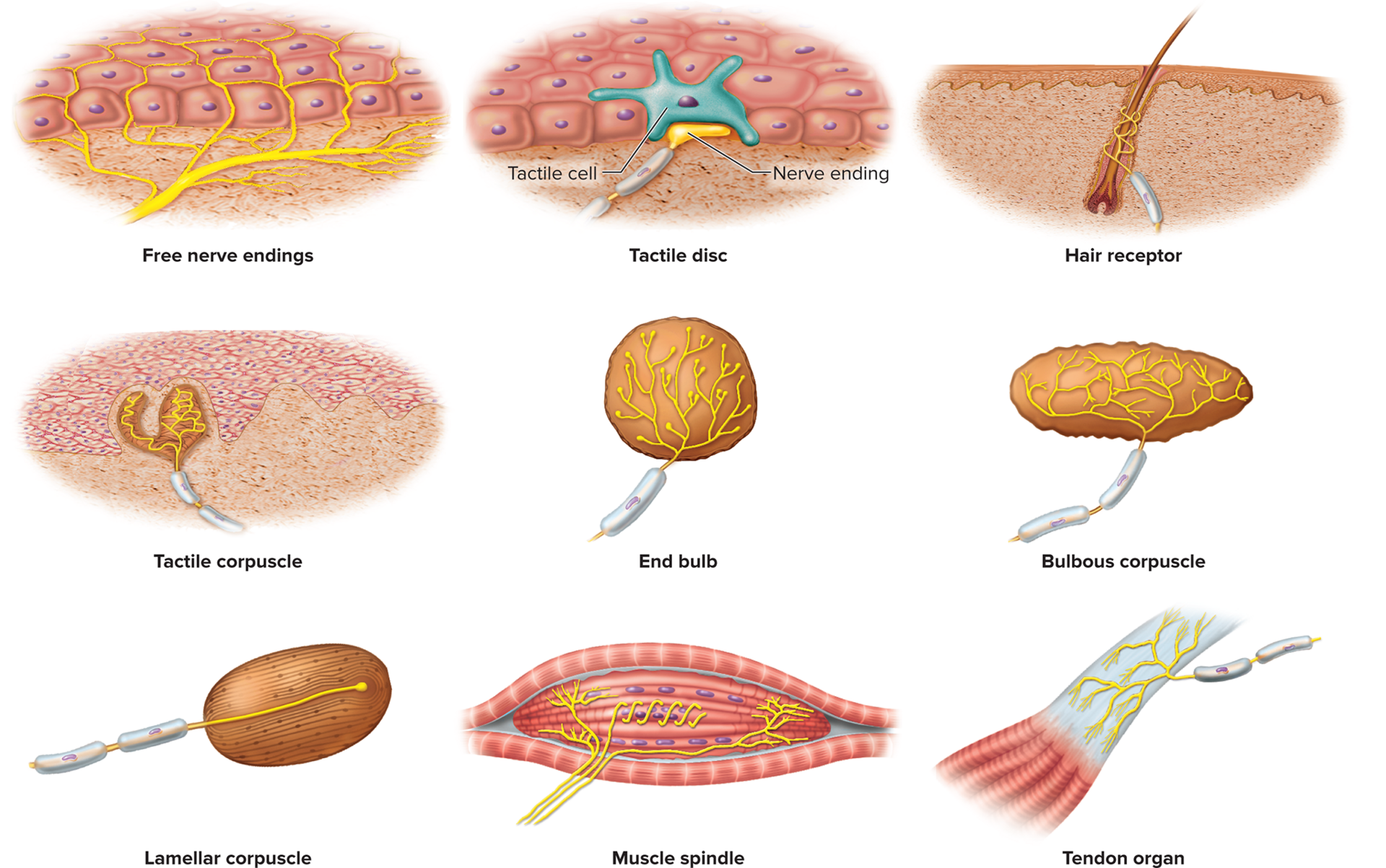
Unencapsulated nerve endings (dendrites with no connective tissue wrapping)
Free nerve endings: simple, bare dendrites abundant in the skin and mucous membranes. They primarily detect temperature (warm/cold) and pain (nociceptors), but also light touch.
Tactile (Merkel) discs: flattened nerve endings that terminate at specialized tactile cells (Merkel cells) in the basal layer of the epidermis. They detect light touch, texture, edges, and shapes, providing sustained light touch and pressure sensation.
Hair receptors (root hair plexuses): dendrites wrapped around the base of hair follicles. They respond to hair movements, sensing light touch and the displacement of hairs.
Encapsulated nerve endings
Nerve fibers wrapped in glial cells or connective tissue; this wrapping often enhances the sensitivity or selectivity of the receptor.
Tactile (Meissner) corpuscles: consist of 2–3 nerve fibers encapsulated in a fluid-filled elliptical capsule. They are rapidly adapting receptors that detect light touch, low-frequency vibration, and texture. They are highly concentrated at the tips of dermal papillae, especially in highly sensitive areas like fingertips, palms, eyelids, nipples, and genitals.
End bulbs (Krause end bulbs): similar in structure to tactile corpuscles, with a sensory nerve fiber surrounded by a connective tissue sheath. Found primarily in mucous membranes (e.g., lips, tongue, conjunctiva) and detect light touch, pressure, and possibly temperature.
Bulbous (Ruffini) corpuscles: flattened, elongated capsules, slowly adapting receptors that detect sustained heavy touch, deep pressure, and skin stretch. They are also important for sensing joint movements and maintaining sustained pressure information.
Lamellar (Pacinian) corpuscles: large, ovoid receptors with a single dendrite surrounded by numerous concentric layers of Schwann cells and fibroblasts, resembling an onion. These are rapidly adapting receptors that detect deep pressure, high-frequency vibration, and stretch. They are located in the periosteum of bone, joint capsules, some viscera (e.g., pancreas, mesenteries), and especially in the deep dermis of the hands, feet, and breasts, as well as genitals.
Somatosensory projection pathways
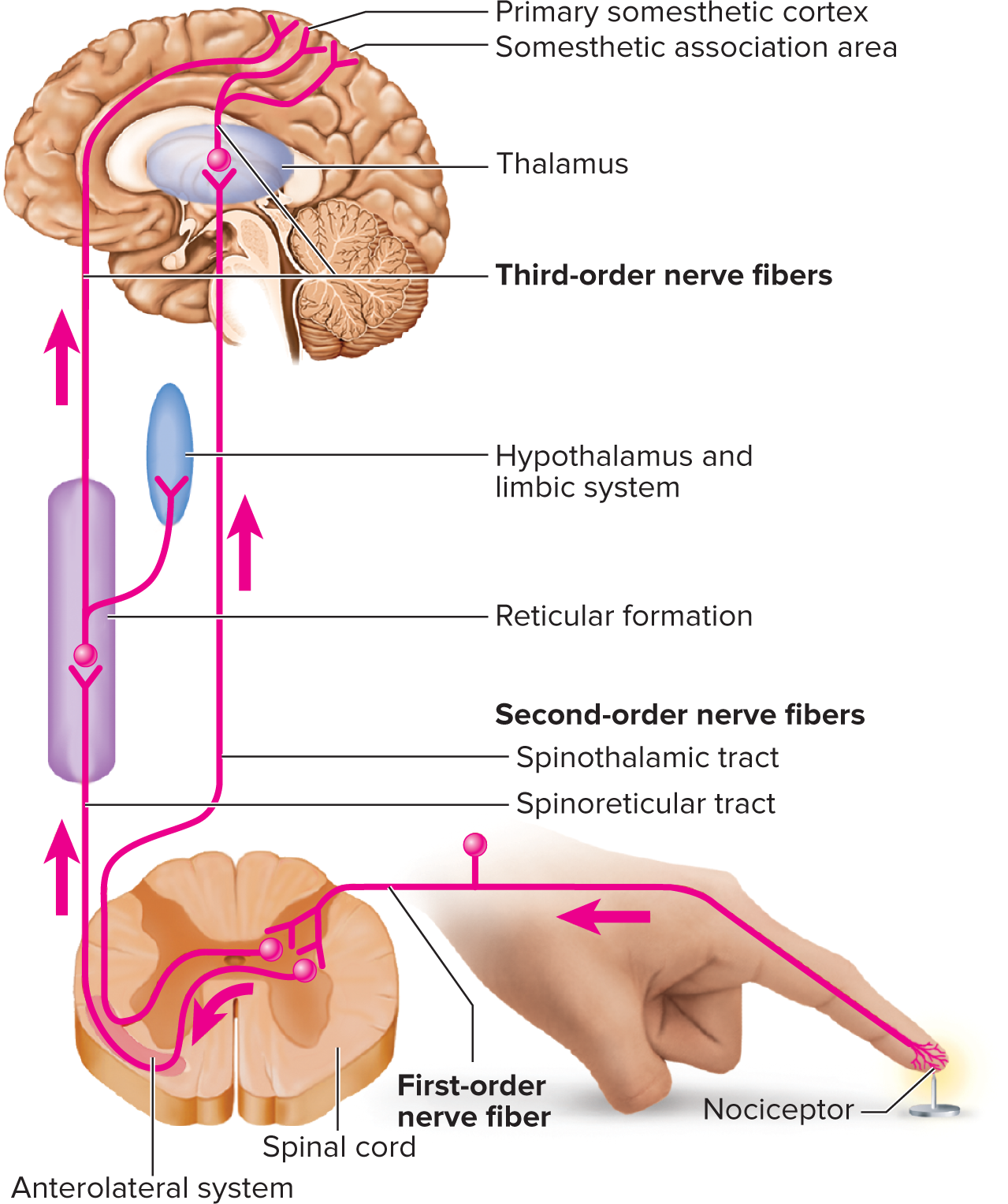
Sensory projection: the transmission of sensory signals from the receptor to the cerebral cortex, typically via a three-neuron pathway.
First-order neuron (primary afferent): carries signals from the receptor to the spinal cord or brainstem. Signals from the head travel to the pons/medulla via cranial nerves (e.g., V, VII, IX, X). Signals from below the head enter the spinal cord via spinal nerves. Touch, pressure, and proprioception fibers (A-beta fibers) are large, heavily myelinated, and fast conducting (v > 30 ext{ m/s}). Heat, cold, and crude touch fibers (A-delta and C fibers) are smaller, less myelinated, or unmyelinated, and conduct slower (v \sim 0.5-30 ext{ m/s}).
Second-order neuron: processes the signal in the spinal cord or brainstem. These neurons decussate (cross) to the opposite side of the CNS in the spinal cord, medulla, or pons. Most second-order neurons from somatic senses (pain, temperature, crude touch, fine touch, vibration, proprioception) ascend to the thalamus, forming pathways like the spinothalamic tract or medial lemniscus. Proprioception signals, however, have a branch that ends in the cerebellum for subconscious motor control.
Third-order neuron: originates in the thalamus. These neurons project from the thalamus to the primary somesthetic cortex (postcentral gyrus) in the cerebrum, where conscious perception and interpretation of the sensation occur.
CNS modulation of pain
Pain perception is influenced by physical and mental state.
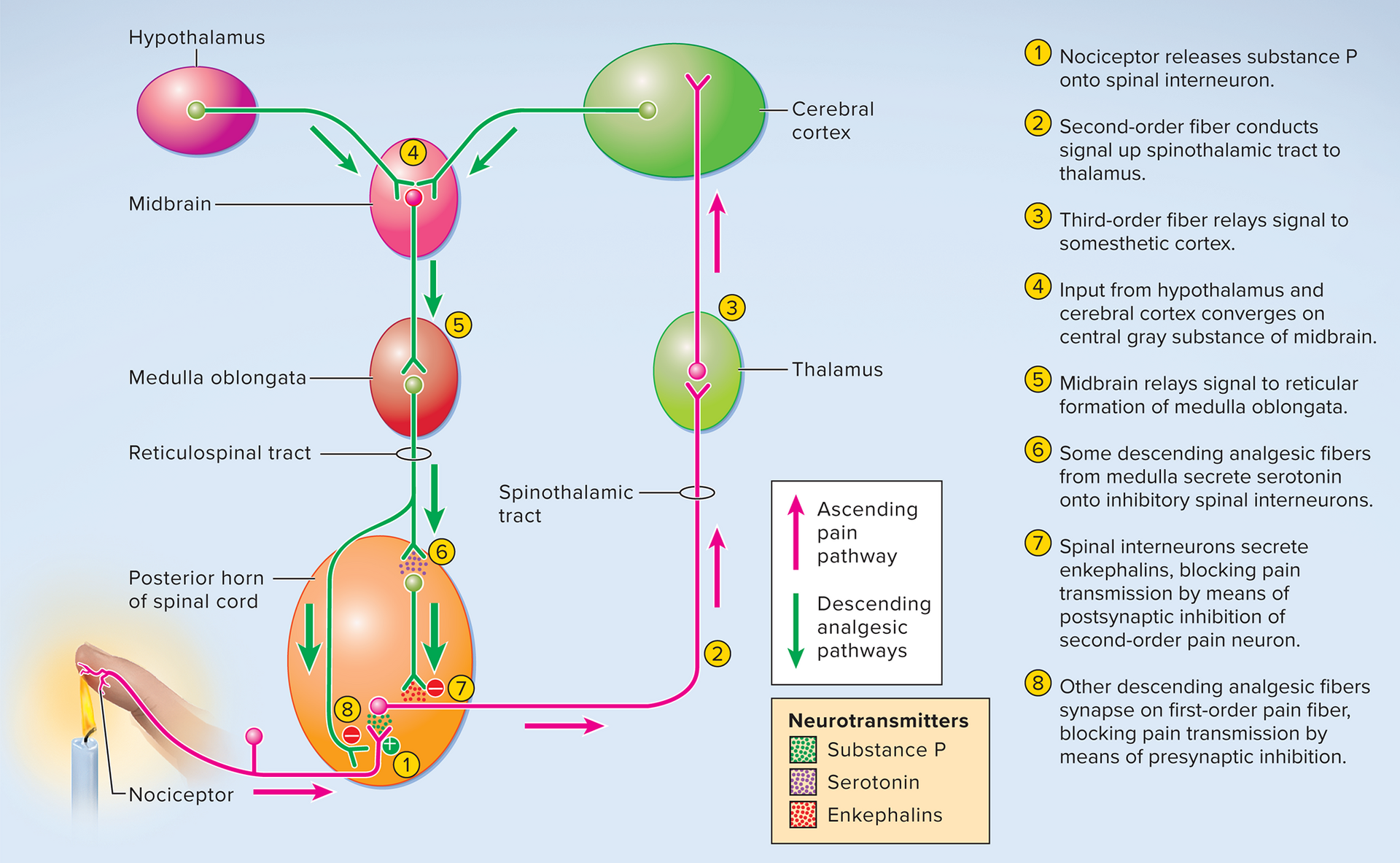
Endogenous Opioids: Natural pain-relieving chemicals (e.g., enkephalins) in CNS bind to opioid receptors, blocking pain transmission.
CNS analgesia mechanisms
Mainly via spinal gating:
Descending fibers from brainstem activate inhibitory spinal interneurons.
These interneurons release enkephalins.
Enkephalins bind to opioid receptors on pain neurons, inhibiting substance P release or hyperpolarizing neurons, thus dampening signals to the brain.
Other gating mechanisms: Mechanoreceptors in skin (e.g., from rubbing) can also activate inhibitory interneurons for pain relief.
Pain (conceptual overview)
Pain: Unpleasant sensory/emotional experience linked to tissue damage; protective function.
Nociceptive pain: From actual/threatened tissue injury, activates nociceptors.
Neuropathic pain: From nerve/CNS injury (e.g., burning, shooting) without apparent tissue damage.
Nociceptive pain subdivided by origin
Visceral pain: From internal organs; diffuse, dull, hard to locate (e.g., due to stretch, ischemia).
Deep somatic pain: From bones, joints, muscles, connective tissues (e.g., arthritis, sprains).
Superficial somatic pain: From skin (e.g., cuts, burns); sharp, well-localized.
Nerve fibers in pain transmission
Fast pain (A-delta fibers): Myelinated, rapid, sharp, stinging, localized (v \text{ up to } 12-30 \text{ m/s}).
Slow pain (C fibers): Unmyelinated, slower, prolonged, burning, dull, diffuse (v \sim 0.5-2 \text{ m/s}).
Projection pathways for pain
Head regions: Pain signals via cranial nerves VII, IX, X to medulla (solitary nucleus) $\rightarrow$ thalamus $\rightarrow$ primary somesthetic cortex.
Neck and below: Via three main ascending spinal cord tracts:
Spinothalamic tract: Most significant pathway for conscious somatic pain and temperature (decussates in spinal cord, ascends to thalamus).
Spinoreticular tract: Carries pain to reticular formation (brainstem), then hypothalamus/limbic system (emotional/autonomic responses).
Gracile fasciculus: Transmits some visceral pain signals from lower body to thalamus.
Nociceptive pain subdivided by origin
Visceral pain: originates from internal organs; it tends to be diffuse, dull, and difficult to precisely locate. Sensations are often described as squeezing, cramping, or deep aching, often accompanied by nausea. It is typically caused by stretch, chemical irritation, or ischemia (lack of blood flow) to the visceral organs.
Deep somatic pain: arises from bones, joints, muscles, and connective tissues (e.g., arthritis, sprains, fractures). It can be caused by excessive stretch (e.g., in an ankle sprain) or direct injury to these deeper structures.
Superficial somatic pain: originates from the skin (e.g., cuts, burns, stings). It is usually sharp, well-localized, and quick to onset.
Nerve fibers in pain transmission
Fast pain (A-delta fibers): transmitted by myelinated A-delta fibers, leading to immediate, sharp, stinging, and highly localized pain sensations (e.g., the initial acute pain from a needle stick). These fibers transmit signals rapidly, with speeds up to v_{ ext{A-delta}} ext{ up to } 12-30 ext{ m/s}.
Slow pain (C fibers): transmitted by unmyelinated C fibers, resulting in a prolonged, burning, dull, aching, and diffuse pain sensation (e.g., the throbbing pain after an injury). These fibers conduct signals much slower, with speeds of approximately v_{ ext{C}} ext{ ~ } 0.5-2 ext{ m/s}.
Projection pathways for pain
Head regions: Pain signals from the head travel via cranial nerves VII (facial), IX (glossopharyngeal), and X (vagus) to the medulla oblongata, where they synapse in the solitary nucleus.
Second-order neurons ascend from the medulla to the thalamus.
Third-order neurons then project from the thalamus to the primary somesthetic cortex in the cerebrum for conscious perception.
Neck and below: Pain signals travel through three main ascending tracts in the spinal cord:
Spinothalamic tract: the most significant pathway for conscious, somatic pain and temperature sensations. Second-order neurons decussate in the spinal cord and ascend to the thalamus.
Spinoreticular tract: carries pain signals to the reticular formation in the brainstem. From here, signals are relayed to the hypothalamus and limbic system, contributing to the emotional and autonomic responses to pain.
Gracile fasciculus: primarily carries fine touch and proprioception from the lower body but also transmits some visceral pain signals to the thalamus, especially from the lower trunk and legs.
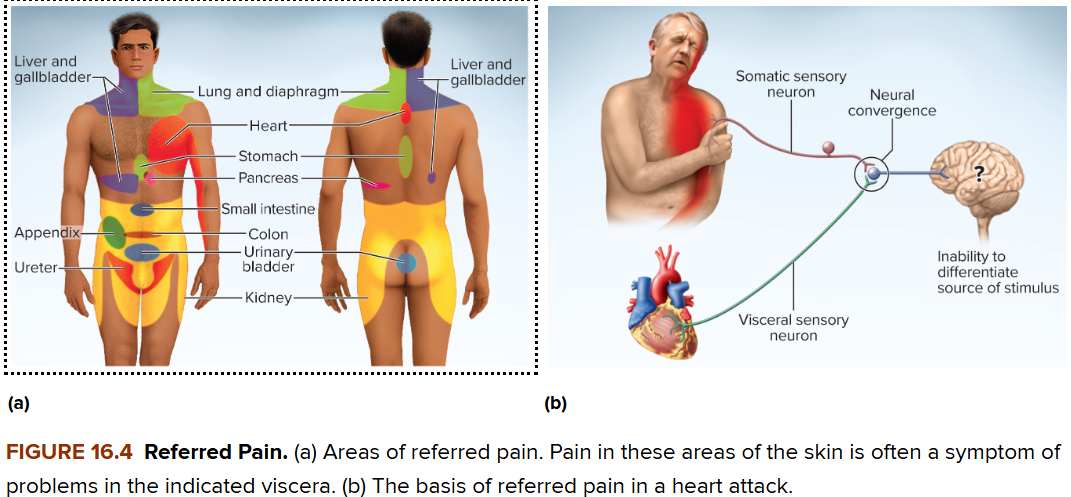
Referred pain
Pain in viscera that is perceived at superficial sites (e.g., skin or muscles) distant from the organ. This phenomenon occurs due to the convergence of visceral and somatic neural pathways onto the same second-order neurons in the spinal cord CNS. The brain, accustomed to receiving signals from the skin, misinterprets the visceral pain as originating from the somatic region.
Example: Heart pain (angina pectoris) is often felt in the left shoulder or arm, and sometimes the jaw or upper back, because the sensory nerves from the heart and those from these somatic areas share spinal cord segments (typically T1–T5).
CNS modulation of pain
Pain perception is influenced by one's physical and mental state (emotions, stress, expectation).
Endogenous opioids: natural pain-relieving chemicals (e.g., enkephalins, endorphins, dynorphins) found in the CNS and other tissues.
They bind to opioid receptors, blocking pain transmission and promoting well-being.
CNS analgesia mechanisms
Pain is mainly blocked via spinal gating:
Descending analgesic fibers from the brainstem send signals down the spinal cord (posterior horn).
These fibers activate inhibitory spinal interneurons, which release enkephalins.
Enkephalins bind to opioid receptors on pain neurons, inhibiting the release of substance P (a pain neurotransmitter) or hyperpolarizing neurons, thus dampening pain signals headed to the brain.
Other gating mechanisms:
Mechanoreceptors in the skin (e.g., from rubbing or massage) can also activate inhibitory spinal interneurons, providing non-pharmacological pain relief (part of the "gate control theory").
CNS analgesia mechanisms
CNS analgesia mechanisms:
Pain is mainly blocked through spinal gating:
Descending fibers from the brainstem travel down to the spinal cord.
These fibers activate inhibitory interneurons in the spinal cord.
These interneurons release enkephalins, which are natural opioids.
Enkephalins bind to opioid receptors on pain-carrying neurons, either inhibiting the release of substance P (a pain neurotransmitter) or making the neurons less excitable.
This dampens or blocks pain signals from reaching the brain.
Other gating mechanisms:
Mechanoreceptors in the skin (e.g., activated by rubbing or massage) can also stimulate
Gating mechanisms also include:
Descending analgesic fibers: originate in midbrain (e.g., periaqueductal gray matter), pons (e.g., reticular formation), and medulla (e.g., nucleus raphe magnus); they descend in the reticulospinal tract and activate inhibitory spinal interneurons in the posterior horn.
Mechanoreceptors in the skin and deeper tissues (e.g., in response to rubbing or massage after an injury) can also activate inhibitory spinal interneurons, providing a non-pharmacological means of pain relief (e.g., the "gate control theory").
16.3 The Chemical Senses
16.3a Gustation—The Sense of Taste
Gustation: the perception of molecules dissolved in water or saliva, known as tastants. Tastants are detected by specialized sensory cells located within taste buds.
chemical stimulants (tastants) detected by sensory cells clustered in 4,000 taste buds
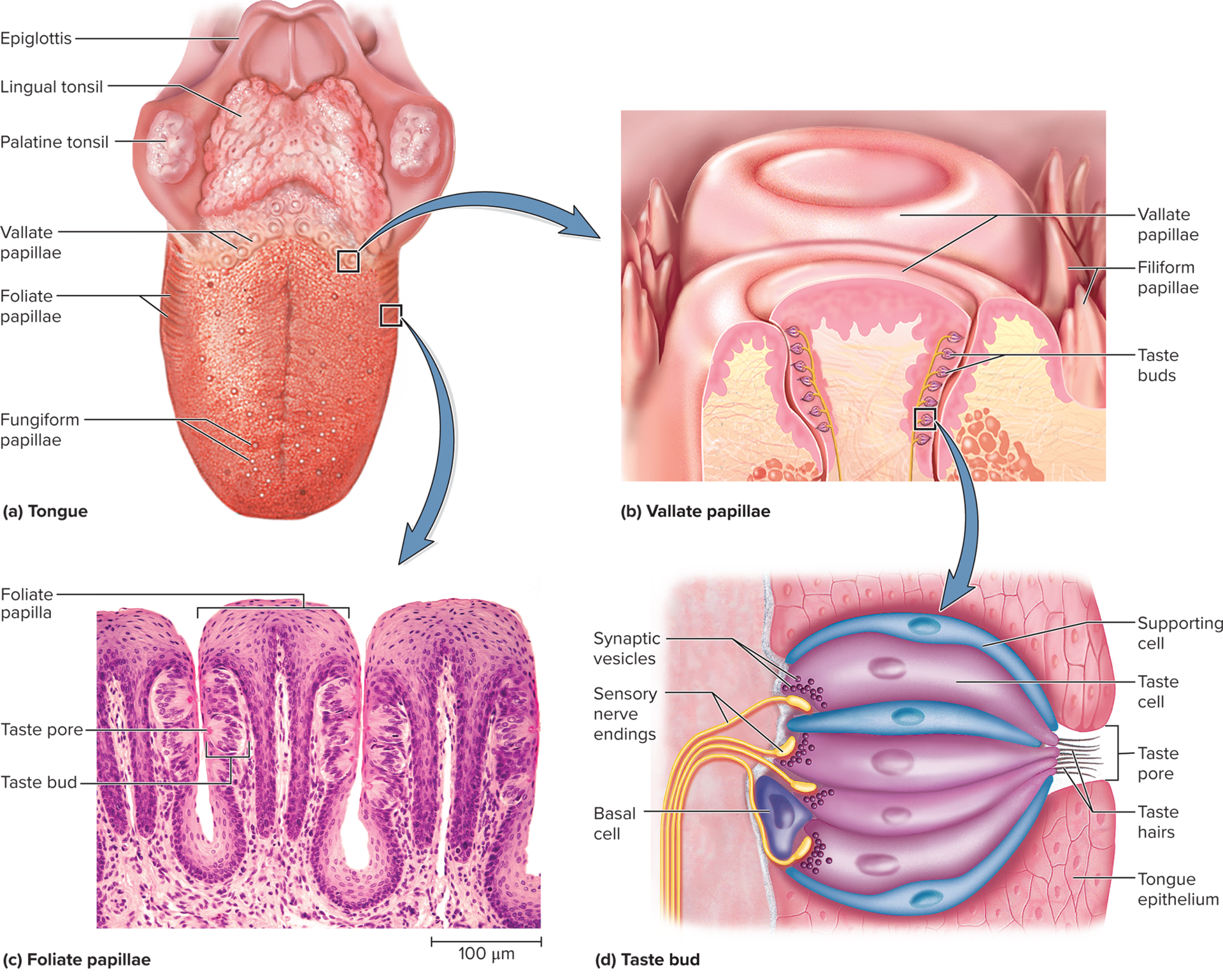
Taste buds: ovoid clusters of 50–100 taste cells (gustatory cells), supporting cells, and basal cells; these are not neurons but specialized epithelial cells.
Lingual papillae: visible bumps on the tongue's surface; there are four types, but only three contain taste buds.
Taste buds reside beneath the epithelial surface of the papillae; taste buds themselves are microscopic and not visible to the naked eye.
Lingual papillae types:
Filiform: small, spiky structures, most abundant type on the tongue. They lack taste buds and are primarily responsible for sensing food texture (mouthfeel) due to their keratinized tips.
Foliate: parallel ridges located along the sides of the posterior tongue. They contain taste buds primarily in children, but these often degenerate by age ~3.
Fungiform: mushroom-shaped papillae scattered over the tips and sides of the tongue. Each fungiform papilla typically contains ~3 taste buds, mainly on its apical surface.
Vallate (circumvallate): largest papillae, arranged in a V-shape at the rear of the tongue. They form a wall-like structure and collectively contain up to half of all taste buds (approximately 250 taste buds per papilla).
Taste cells: specialized epithelial cells with an apical microvilli (taste hairs) that project into a taste pore, allowing them to bind with tastants. These cells synapse with dendrites of sensory neurons at their base.
Basal cells: stem cells located at the base of the taste bud that divide and differentiate to replace taste cells, which have a lifespan of only 7–10 days.
Supporting cells: resemble taste cells but lack synaptic vesicles and do not appear to have a direct role in taste sensation.
16.3a Taste physiology
Five primary tastes (taste modalities), each detected by distinct mechanisms:
Salty: primarily detected by the presence of metal ions (e.g., Na+, K+). Sodium ions enter taste cells directly through ion channels, causing depolarization.
Sweet: typically indicates carbohydrates and other foods with caloric value. Detected by sugars (e.g., glucose, sucrose) and artificial sweeteners, which bind to G protein-coupled receptors.
Umami: a savory or "meaty" taste, associated with amino acids such as glutamate and aspartate (found in chicken/beef broth, aged cheese, soy sauce). Also detected by G protein-coupled receptors.
Sour: caused by acids (e.g., citric acid in lemons). Hydrogen ions (H+) from acids enter taste cells directly through ion channels or block K+ channels.
Bitter: often indicates spoiled foods or potentially toxic alkaloids (e.g., nicotine, caffeine, quinine, aspirin). Detected by a diverse family of G protein-coupled receptors, which provides a protective mechanism against poisons.
Two additional proposed primary tastes: Oleogustus (the taste of fatty acids) and Water (a distinct sensation perceived by some animals and potentially humans).
Taste is strongly influenced by other sensory inputs, including food texture (mouthfeel, detected primarily by the lingual nerve branches in the papillae), aroma (smell, contributing significantly to overall flavor perception), temperature, and appearance.
Capsaicin (found in hot peppers) and other pungent compounds do not stimulate taste buds directly but activate nociceptors (pain receptors) in the mouth via the trigeminal nerve, creating a sensation of spiciness or heat rather than taste.
16.3a Taste transduction
Two main mechanisms for taste cell stimulation:
Second-messenger systems: Sugars (sweet), alkaloids (bitter), and glutamate/aspartate (umami) activate G protein-coupled receptors on the taste cell membranes. This binding initiates a second-messenger cascade (e.g., involving cAMP or IP3/DAG) within the cell, leading to depolarization.
Direct ion entry: Sodium ions (salty) and hydrogen ions (sour) enter taste cells directly through specific ion channels or by blocking K+ channels, causing immediate depolarization of the cell membrane.
Both mechanisms lead to depolarization of the taste cell, which triggers the release of neurotransmitters (e.g., ATP, serotonin) at the base of the taste cell. These neurotransmitters then stimulate the dendrites of associated sensory neurons.
Taste cells within a taste bud exhibit local processing of gustatory information and may communicate via gap junctions. Some taste cells also secrete hormones to signal digestive organs about incoming food, initiating preparatory physiological responses.


6.3a Gustation projection pathways
Three cranial nerves carry taste information from the tongue and oral cavity:
Facial nerve (CN VII): collects taste signals from the anterior two-thirds of the tongue.
Glossopharyngeal nerve (CN IX): innervates the posterior one-third of the tongue.
Vagus nerve (CN X): carries taste signals from the palate, pharynx, and epiglottis.
All three cranial nerves synapse in the solitary nucleus of the medulla oblongata.
Second-order neurons relay signals from the solitary nucleus to two main destinations:
Hypothalamus and amygdala: These connections are involved in taste-related reflexes such as salivation, gagging, and vomiting, as well as emotional responses to taste and appetite regulation.
Thalamus: Specifically, the ventral posterior nucleus of the thalamus.
Third-order neurons project from the thalamus to the primary gustatory cortex, located in the insula of the cerebral cortex, where conscious perception of taste occurs. Signals also project to the orbitofrontal cortex, where taste, smell, and textural information are integrated to form the overall perception of flavor and palatability.
16.3b Olfaction—The Sense of Smell
Olfaction: the response to airborne chemicals called odorants, perceived as smells.
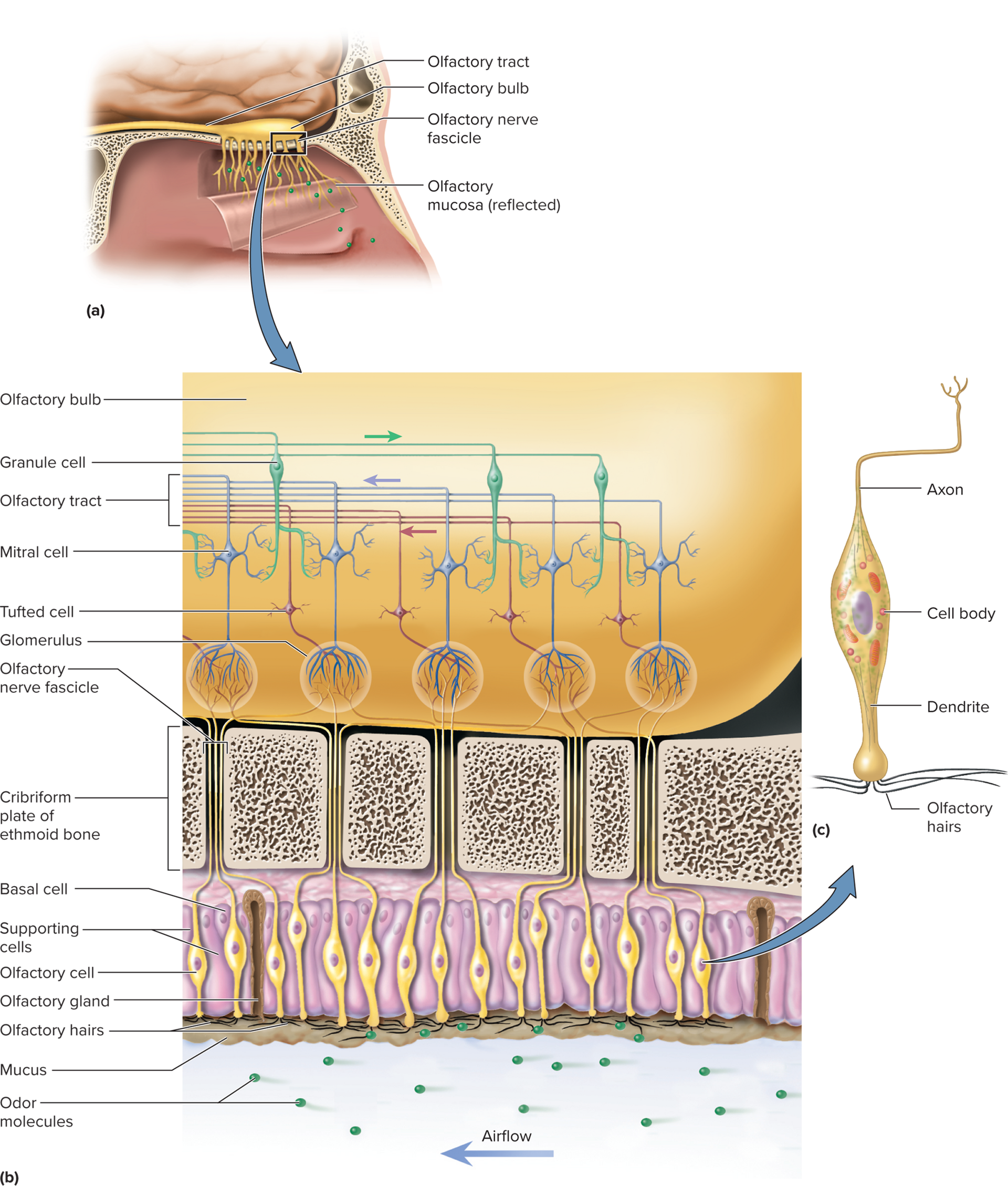
Olfactory mucosa: a specialized patch of pseudostratified epithelium located at the roof of the nasal cavity. It houses the olfactory receptor cells, supporting cells, and basal stem cells.
Covers part of the superior nasal concha, the cribriform plate of the ethmoid bone, and the superior part of the nasal septum in each nasal fossa.
Olfactory cells:
Unique neurons specialized for odorant detection.
Dendrites extend to epithelium surface with 10–20 immobile cilia (olfactory hairs).
Axons form olfactory nerve (CN I), passing through cribriform plate to olfactory bulbs.
Humans have ~400 odorant receptor types; each olfactory cell expresses only one specific type.
Odors identified by brain based on unique combinatorial activation patterns of these receptors.
Odorant binding & transduction:
Odorant binds to G protein-coupled receptors on olfactory cilia.
Initiates cAMP second messenger cascade
Opens ion channels (e.g., Na+, Ca2+), causing depolarization and action potential generation.
Irritant odorants:
Certain irritants (e.g., ammonia, capsaicin) activate nociceptors of the trigeminal nerve (CN V) directly.
Produce sensations like stinging or burning, distinct from true smell.
16.3b Olfaction projection pathways:
First synapse: Olfactory cell axons pass through cribriform plate and synapse with mitral and tufted cells within spherical glomeruli in the olfactory bulbs.
Each glomerulus receives input exclusively from olfactory cells expressing the same odorant receptor type, creating a spatial map.
Higher brain centers interpret complex odors by combining activity across multiple glomeruli.
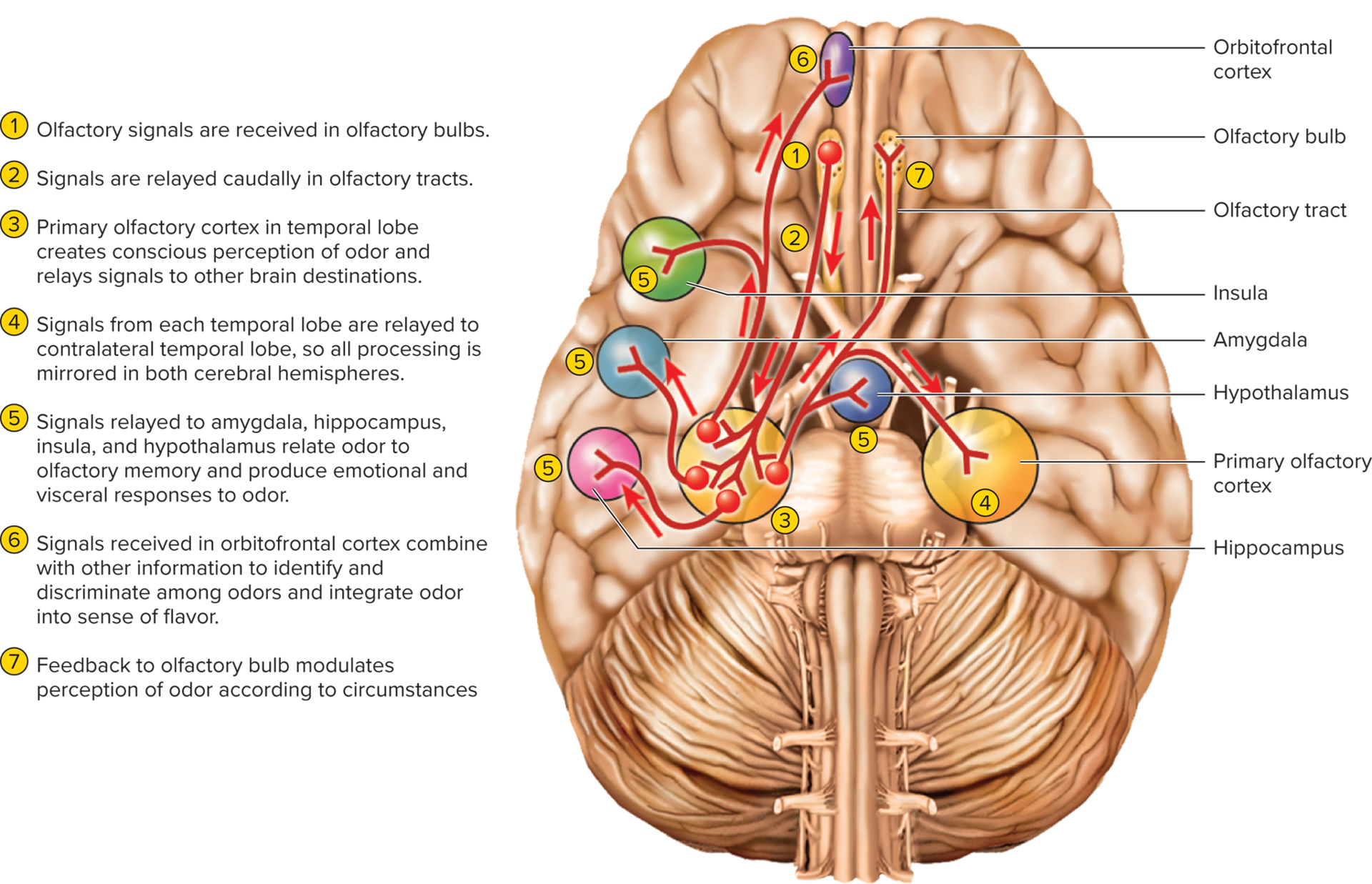
Primary cortical projection: Output from olfactory bulbs travels via olfactory tracts directly to the primary olfactory cortex (piriform cortex and amygdala) in the inferior and medial temporal lobe.
Note: Unlike other senses, olfaction typically bypasses direct thalamic relay to its primary cortical area, though subsequent relays may involve the thalamus.
Limbic system connections: Signals from the primary olfactory cortex also project directly to the amygdala, hippocampus (parts of the limbic system), insula, and hypothalamus.
These strong ties explain why smells often evoke powerful memories, emotional responses, and influence appetite and behavior.
16.3b Olfaction projection pathways
Olfactory cell axons pass through the cribriform plate and synapse with mitral and tufted cells within spherical structures called glomeruli in the olfactory bulbs. Each glomerulus receives input exclusively from olfactory cells that express the same type of odorant receptor, creating a spatial map of odors.
Higher brain centers interpret complex odors by combining the activity patterns across multiple glomeruli.
Output from the olfactory bulbs travels via the olfactory tracts directly to the primary olfactory cortex, located in the inferior and medial temporal lobe (piriform cortex and amygdala).
Unlike other senses, olfaction does not typically synapse in the thalamus before reaching its primary cortical area. However, there are subsequent relays back to the thalamus for further processing.
Some signals from the primary olfactory cortex directly project to the amygdala and hippocampus (parts of the limbic system), insula, and hypothalamus.
These strong ties to limbic structures explain why smells often evoke powerful memories, emotional responses, and influence appetite and behavior.
16.4 Hearing and Equilibrium
16.4a The Nature of Sound
Sound: an audible vibration of molecules (a disturbance that travels through a medium).
A vibrating object (source) pushes on air molecules, creating alternating regions of high pressure (compressions) and low pressure (rarefactions), which then push on others, transmitting the sound wave. This causes the eardrum to vibrate in response to these air pressure changes.
Physical properties of sound waves:
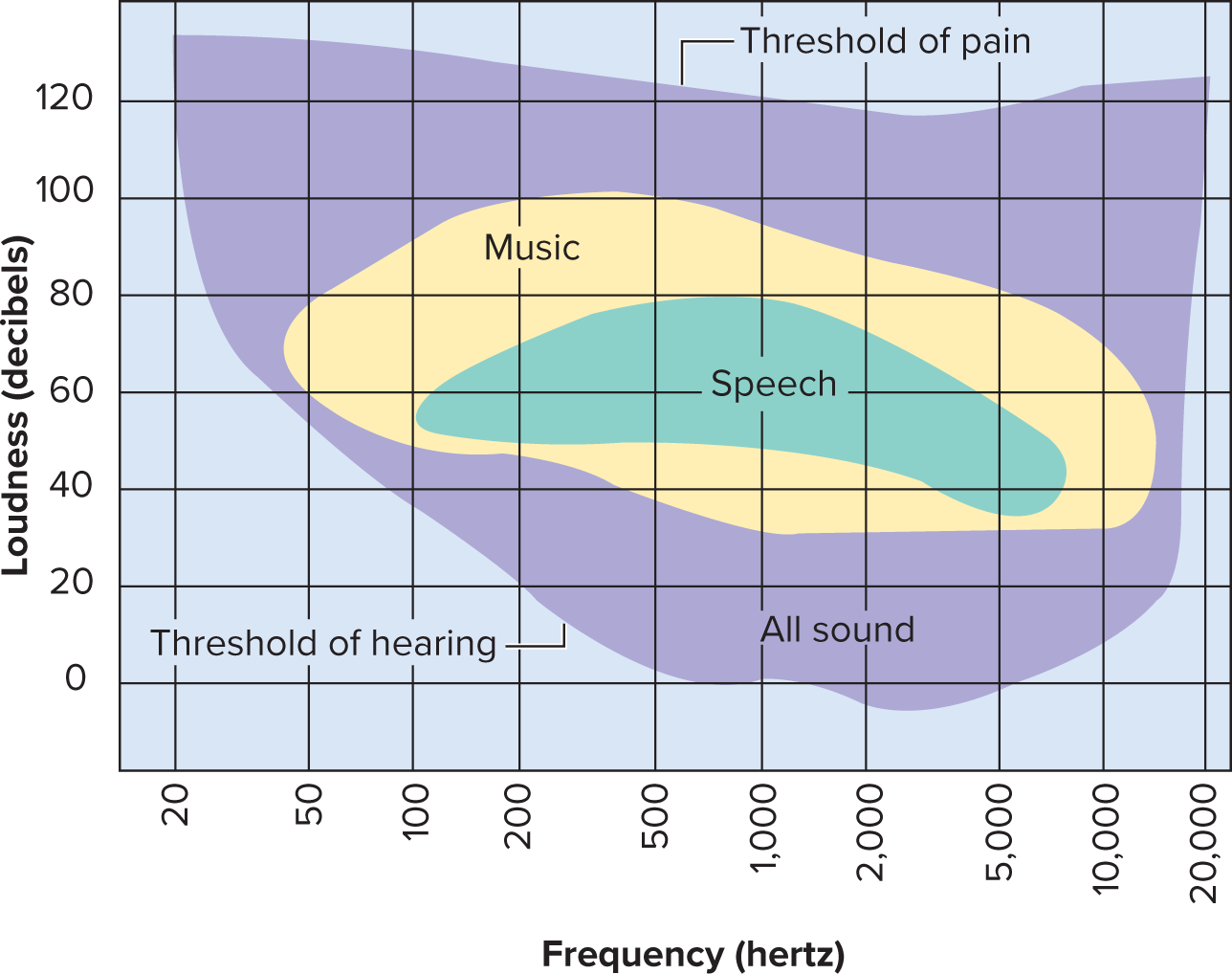
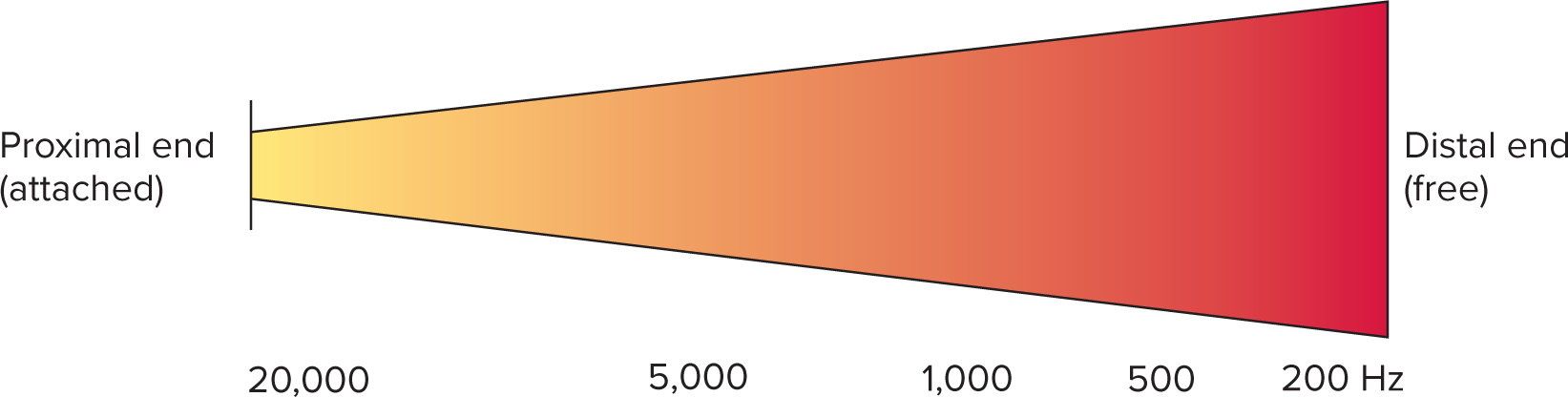
Pitch: determined by the frequency of vibration, measured in Hertz (Hz). Higher frequency corresponds to higher pitch.
humans can hear from 20 to 20,000 Hz
Loudness: the perception of sound energy or amplitude, measured in decibels (dB).
Greater amplitude corresponds to louder sound.
Normal conversation is approximately 60 dB. The threshold for pain is typically around 120 to 140 dB
16.4b Anatomy of the Ear
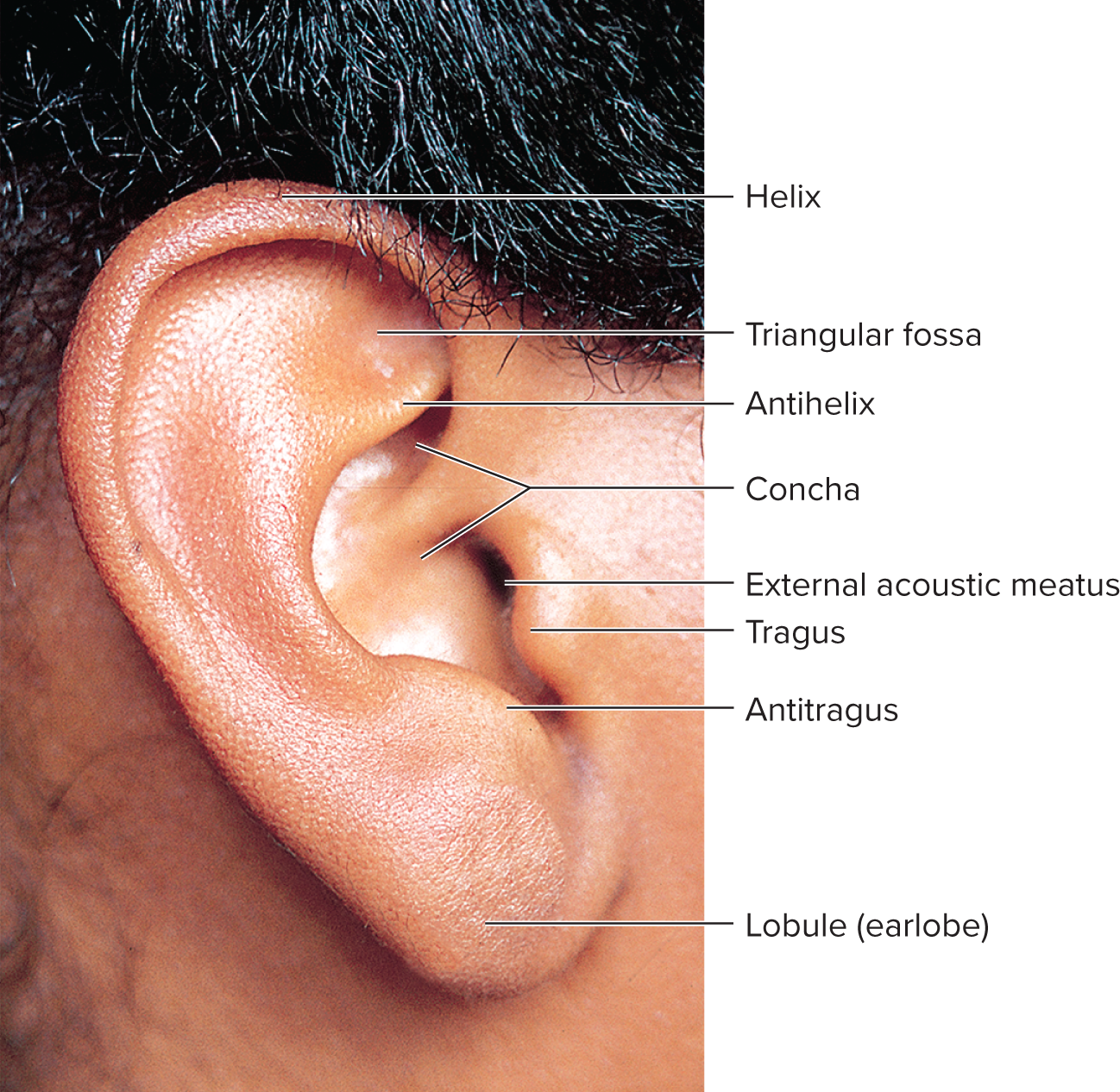
Outer ear: designed as a funnel for vibrations and protection.
Pinna (auricle): the visible fleshy part of the ear, which funnels sound waves into the external acoustic meatus.
External acoustic meatus (ear canal): a tube about 3 cm long extending from the auricle to the eardrum. It contains:
Ceruminous and sebaceous glands: produce cerumen (earwax), a sticky substance that traps dust, foreign particles, and acts as a lubricant and antibacterial agent.
Guard hairs: protect the canal opening from insects and debris.
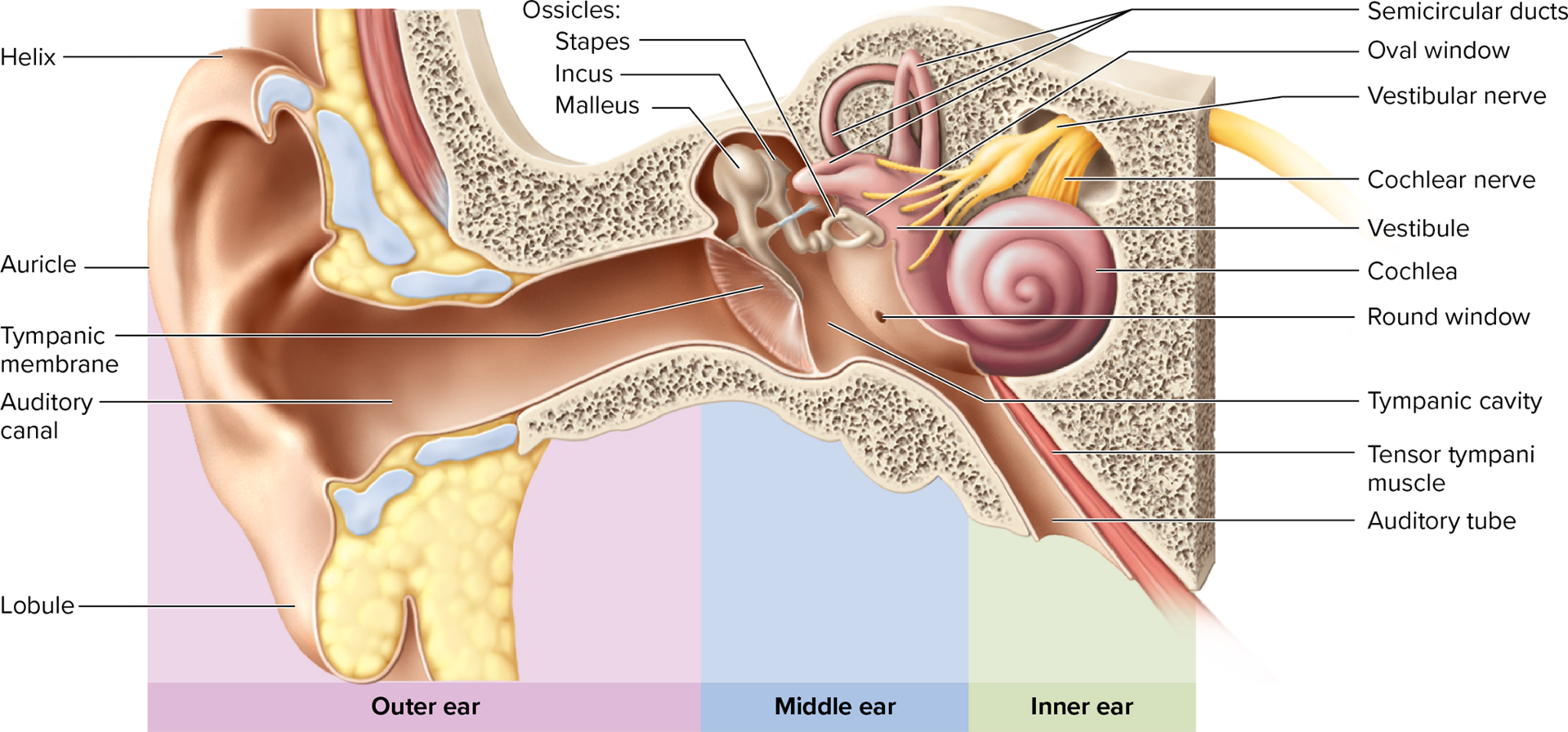
Middle ear: an air-filled tympanic cavity located within the temporal bone.
Tympanic membrane (eardrum): a thin, oval, slightly conical membrane that vibrates in response to sound waves. It is innervated by branches of the vagus (CN X) and trigeminal (CN V) nerves, making it very sensitive.
Auditory (eustachian/pharyngotympanic) tube: connects the middle ear to the nasopharynx. Its primary function is to equalize air pressure between the middle ear and the atmosphere, allowing the eardrum to vibrate freely.
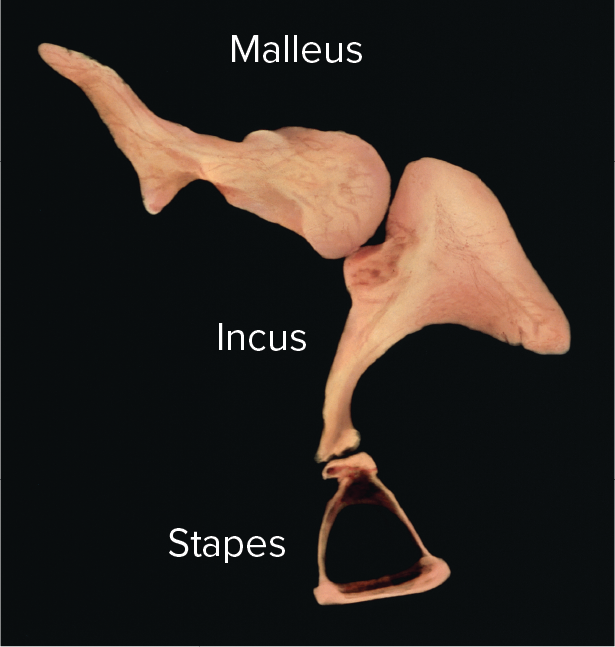
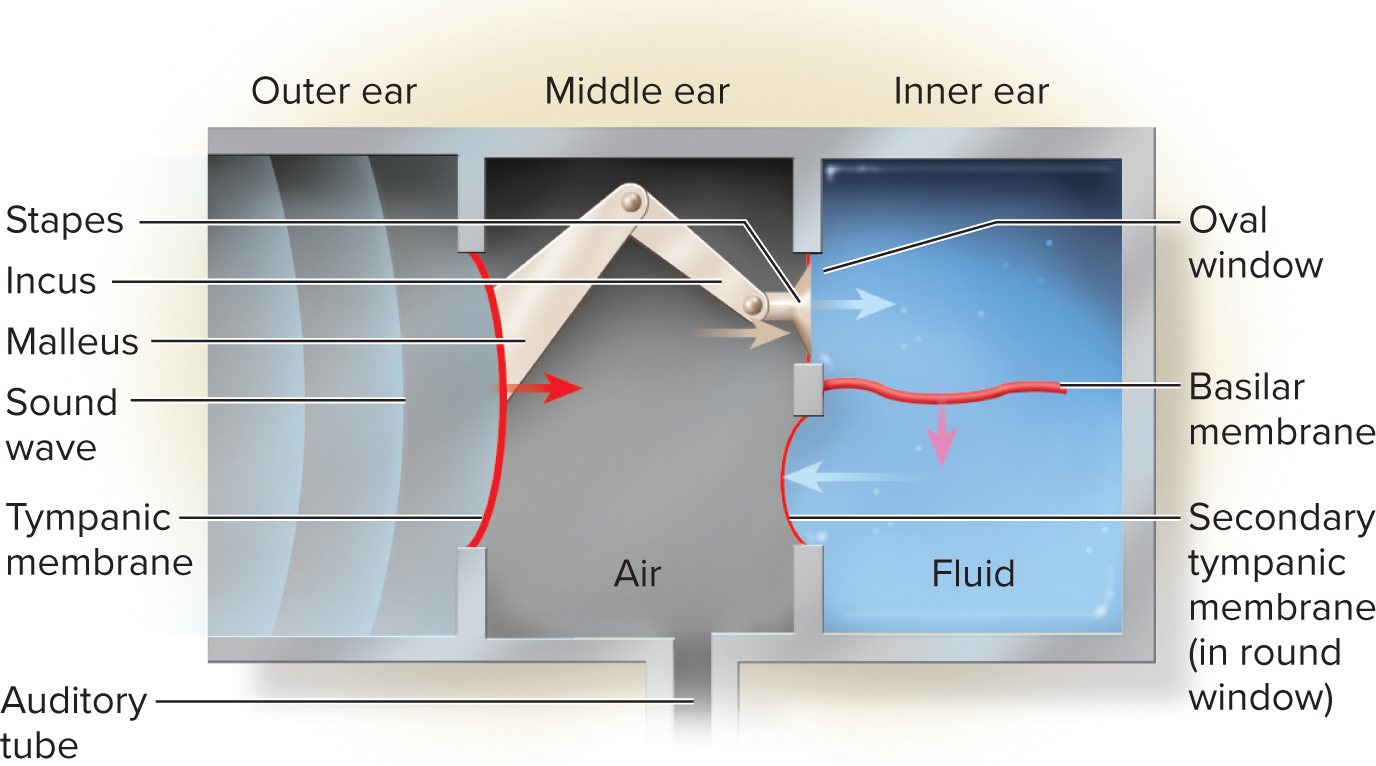
Auditory ossicles: the three smallest bones in the body, which form a lever system to transmit and amplify vibrations from the eardrum to the inner ear's oval window.
Malleus (hammer): attached to the eardrum.
Incus (anvil): articulates with the malleus and stapes.
Stapes (stirrup): nested in the oval window of the inner ear.
Stapedius and tensor tympani muscles: two small muscles that protect the inner ear by contracting reflexively (attenuation reflex) in response to loud sounds, thereby damping the vibrations of the ossicles and reducing the transmission of excessive sound energy.
Middle-ear infection (otitis media): common in children due to their shorter and more horizontal auditory tube, which allows easier spread of pathogens from the throat. Symptoms include fluid accumulation in the tympanic cavity, pain, and hearing loss. In severe or recurrent cases, a tympanostomy (insertion of a tube in the eardrum) may be performed to drain fluid and equalize pressure.
16.4c The Physiology of Hearing
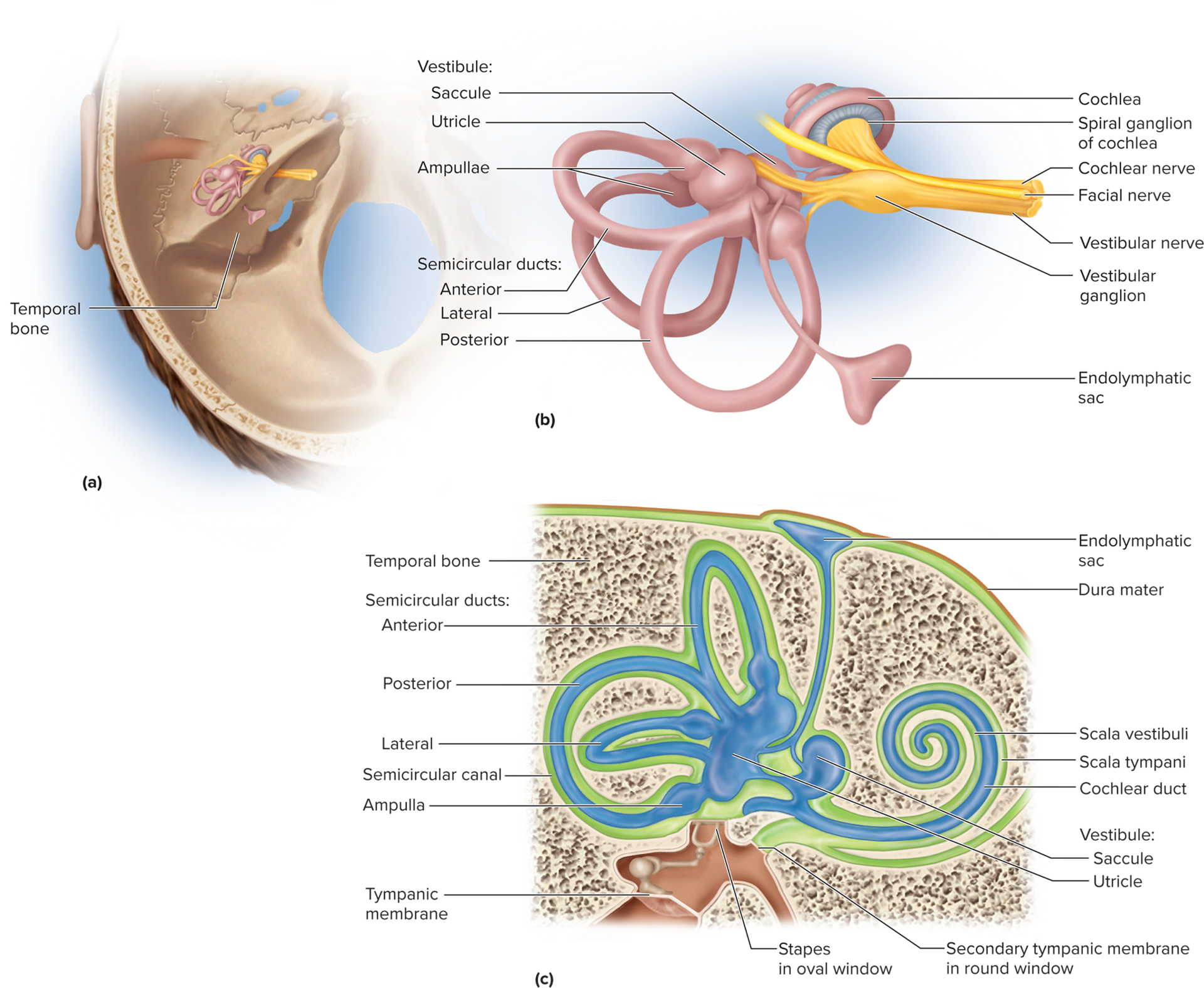
Inner ear anatomy: located within the skull's temporal bone, consisting of:
Bony labyrinth: a maze of passages within the temporal bone (cochlea, vestibule, semicircular canals) filled with perilymph (a fluid similar to CSF).
Membranous labyrinth: a series of fluid-filled sacs and ducts within the bony labyrinth, filled with endolymph (a unique fluid with a high K+ and low Na+ concentration, essential for hair cell function).
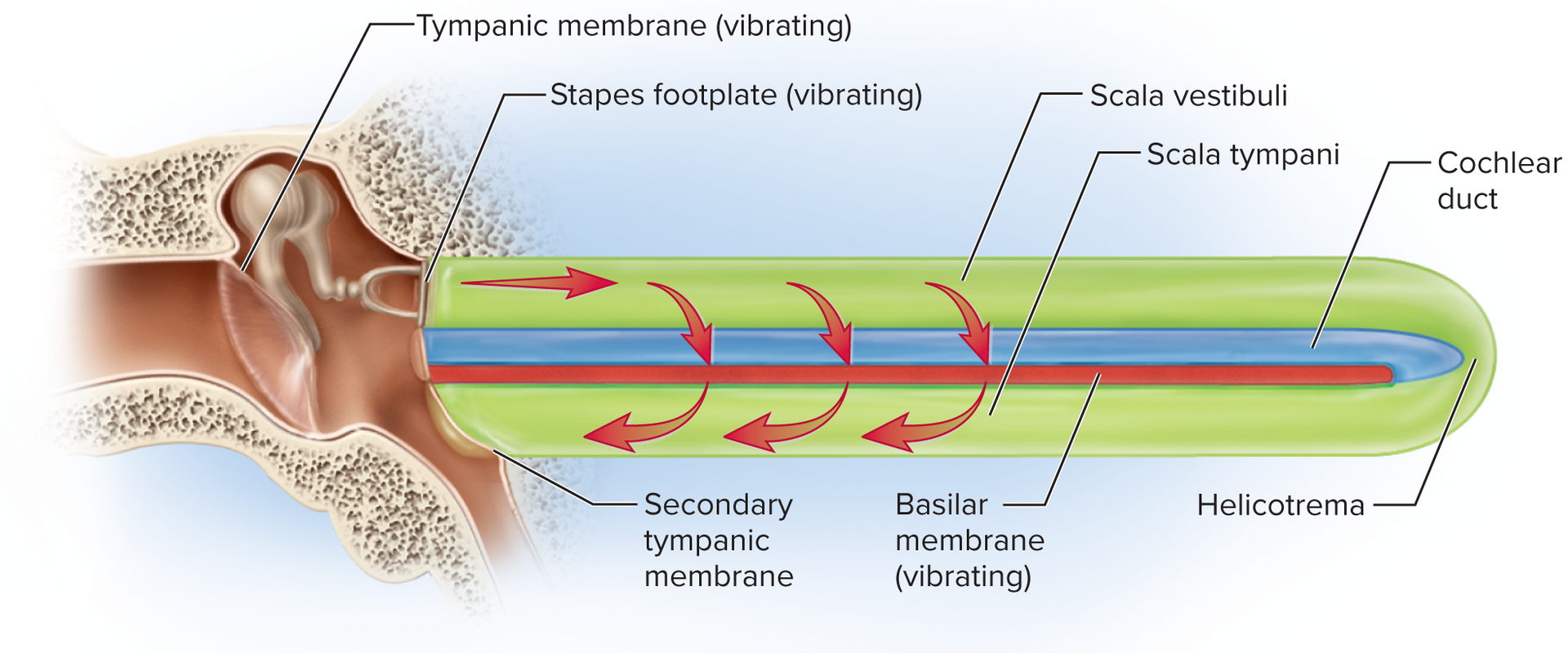
Cochlea: the snail-shaped portion of the inner ear primarily responsible for hearing. It contains three fluid-filled ducts:
Scala vestibuli: the superior chamber, filled with perilymph, continuous with the oval window.
Scala tympani: the inferior chamber, filled with perilymph, continuous with the round window.
Cochlear duct/scala media: the middle chamber, filled with endolymph. This is the true "organ of hearing."
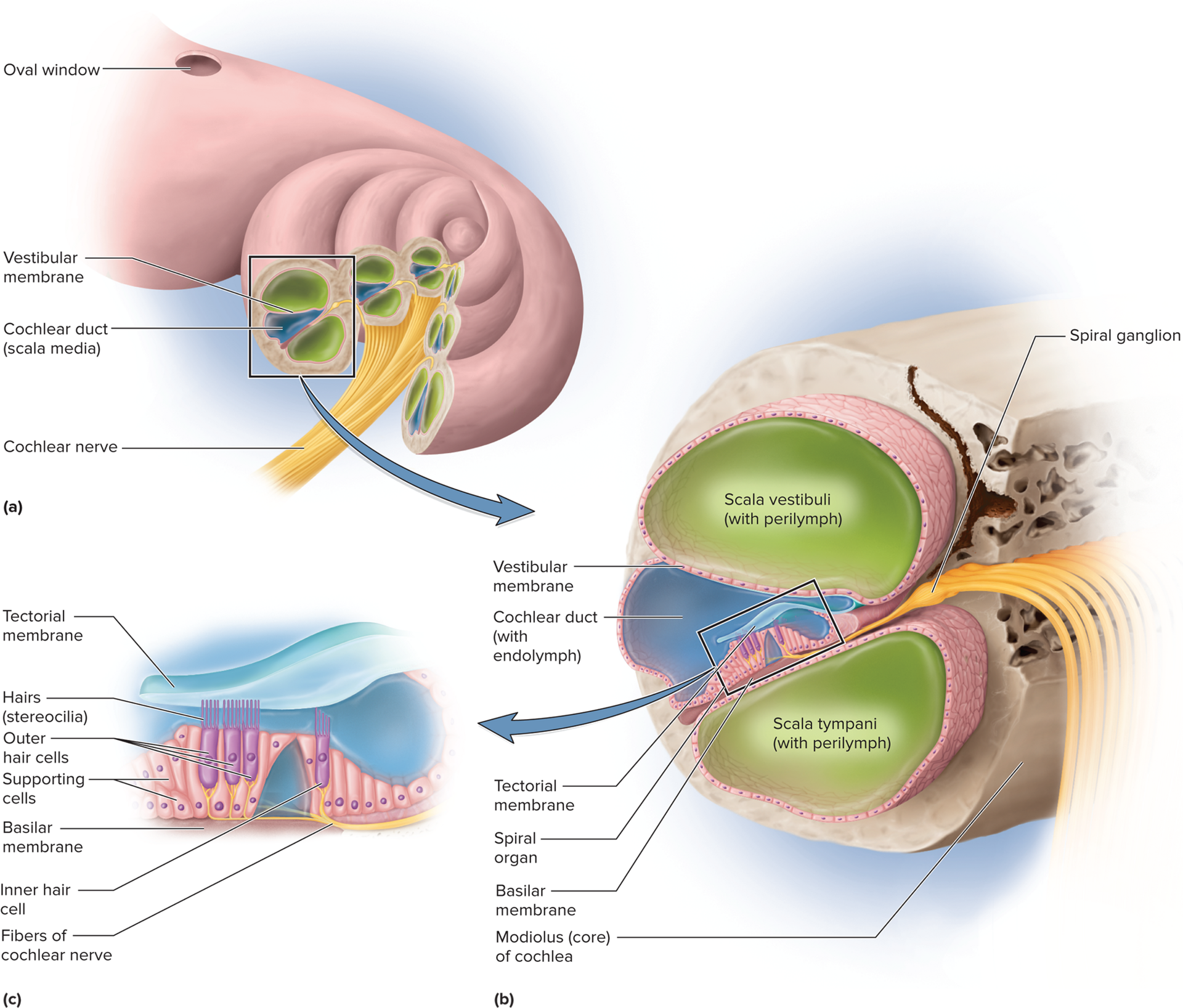
Spiral organ (organ of Corti): the sensory organ for hearing, located within the cochlear duct and resting on the basilar membrane. It contains several rows of hair cells:
Spiral organ (organ of Corti):
Sensory organ for hearing, located within the cochlear duct and resting on the basilar membrane.
Inner hair cells (IHCs): Single row, primary transducers (~90-95% of auditory information to brain).
Outer hair cells (OHCs): Three to five rows, receive efferent input, enhance IHC sensitivity, and sharpen frequency tuning (cochlear tuning).
Hair cell anatomy:
Each hair cell has stereocilia (microvilli-like structures) extending into the endolymph.
Some have a kinocilium (true cilium) for equilibrium (vestibular system).
Tectorial membrane lies above stereocilia; basilar membrane vibration bends stereocilia against it.
Endolymph (high K+ concentration) creates a critical electrochemical gradient for hair cell function.
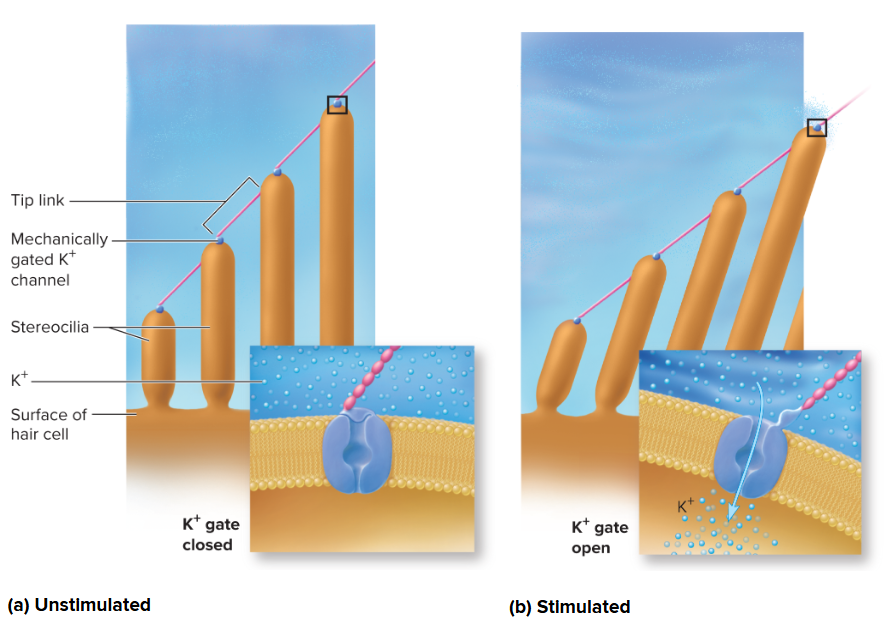
Mechanism of hair-cell transduction:
Sound vibrations transmit to perilymph, causing the basilar membrane to vibrate.
This movement bends hair cell stereocilia against the tectorial membrane (or by endolymph flow).
Bending stresses mechanically gated ion channels (linked by tip links), causing them to open.
K+ ions from endolymph rush into the hair cell, causing depolarization.
Depolarization triggers voltage-gated Ca2+ channels to open, leading to Ca2+ influx.
This results in neurotransmitter (e.g., glutamate) release at the hair cell base, stimulating cochlear nerve neuron dendrites.
Outer hair cells (OHCs): arranged in three to five rows, these cells primarily receive efferent input from the brain and enhance the sensitivity of IHCs and sharpen frequency tuning (cochlear tuning).
Hair cell anatomy: each hair cell possesses stereo-cilia (microvilli-like structures) that extend into the endolymphatic fluid. Some also have a single kinocilium (a true cilium), which plays a role in equilibrium in the vestibular system. The tectorial membrane lies above the stereocilia; when the basilar membrane vibrates, the stereocilia bend against the tectorial membrane. The endolymph bathing the hair cell tops creates a significant electrochemical gradient (due to its high K+ concentration), critical for their function.
Mechanism of hair-cell transduction: Sound vibrations transmitted to the perilymph cause the basilar membrane to vibrate. This movement bends the stereocilia of the hair cells against the tectorial membrane (or directly by endolymph flow). The bending stresses mechanically gated ion channels (linked by tip links), causing them to open. K+ ions (from the endolymph) rush into the hair cell, causing depolarization. This depolarization triggers voltage-gated Ca2+ channels to open, leading to an influx of Ca2+ and subsequent release of neurotransmitters (e.g., glutamate) at the base of the hair cell, stimulating the dendrites of the cochlear nerve neurons.
16.4c The Physiology of Hearing (continued)
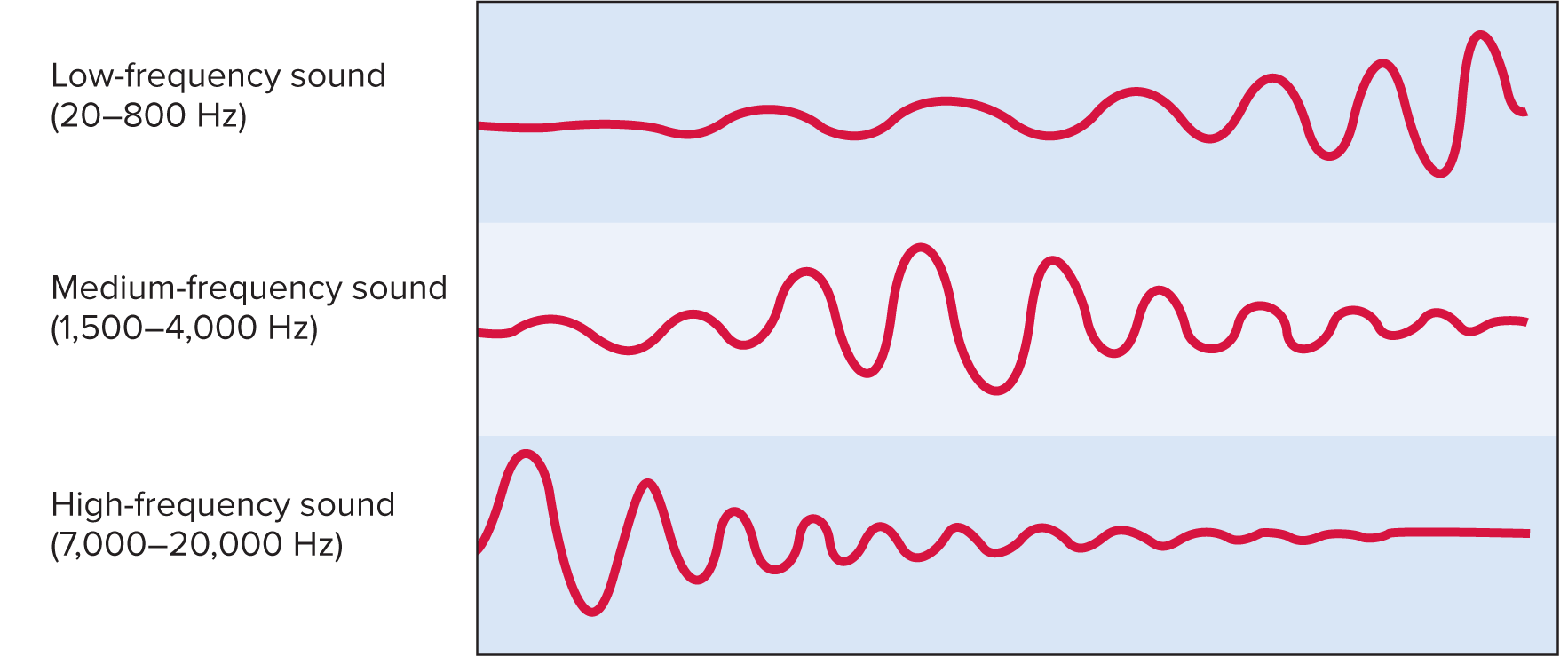
Frequency/pitch mapping ("Place Theory"):
Basilar membrane varies:
Basal (proximal) end: narrow, stiff; responds to high frequencies (f).
Apical (distal) end: wide, floppy; responds to low frequencies (f).
Pitch determined by part of basilar membrane most vigorously stimulated.
Cochlear tuning:
Outer Hair Cells (OHCs) enhance Inner Hair Cell (IHC) sensitivity and sharpen frequency tuning.
OHCs (with efferent input from pons) shorten/lengthen to amplify specific basilar membrane vibrations.
Improves pitch discrimination, especially in noisy environments.
Cochlear tuning: Outer hair cells (OHCs) play a crucial role in sharpening the tuning of the basilar membrane and enhancing the sensitivity of inner hair cells. They receive efferent signals from the pons, allowing them to shorten or lengthen, which amplifies the vibration of specific regions of the basilar membrane at specific frequencies. This active process enhances the contrast between active areas, improving pitch discrimination, especially in noisy environments.
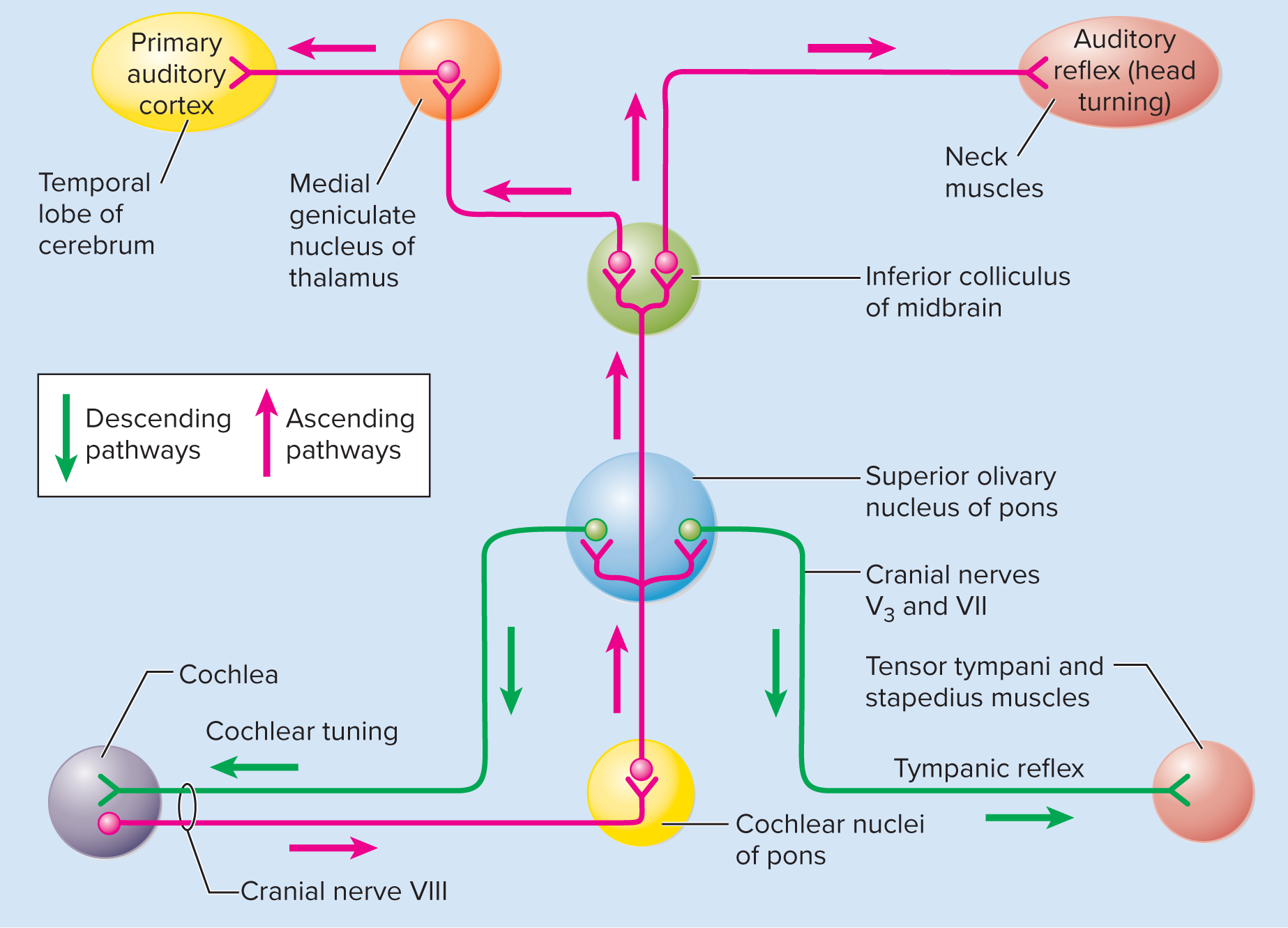
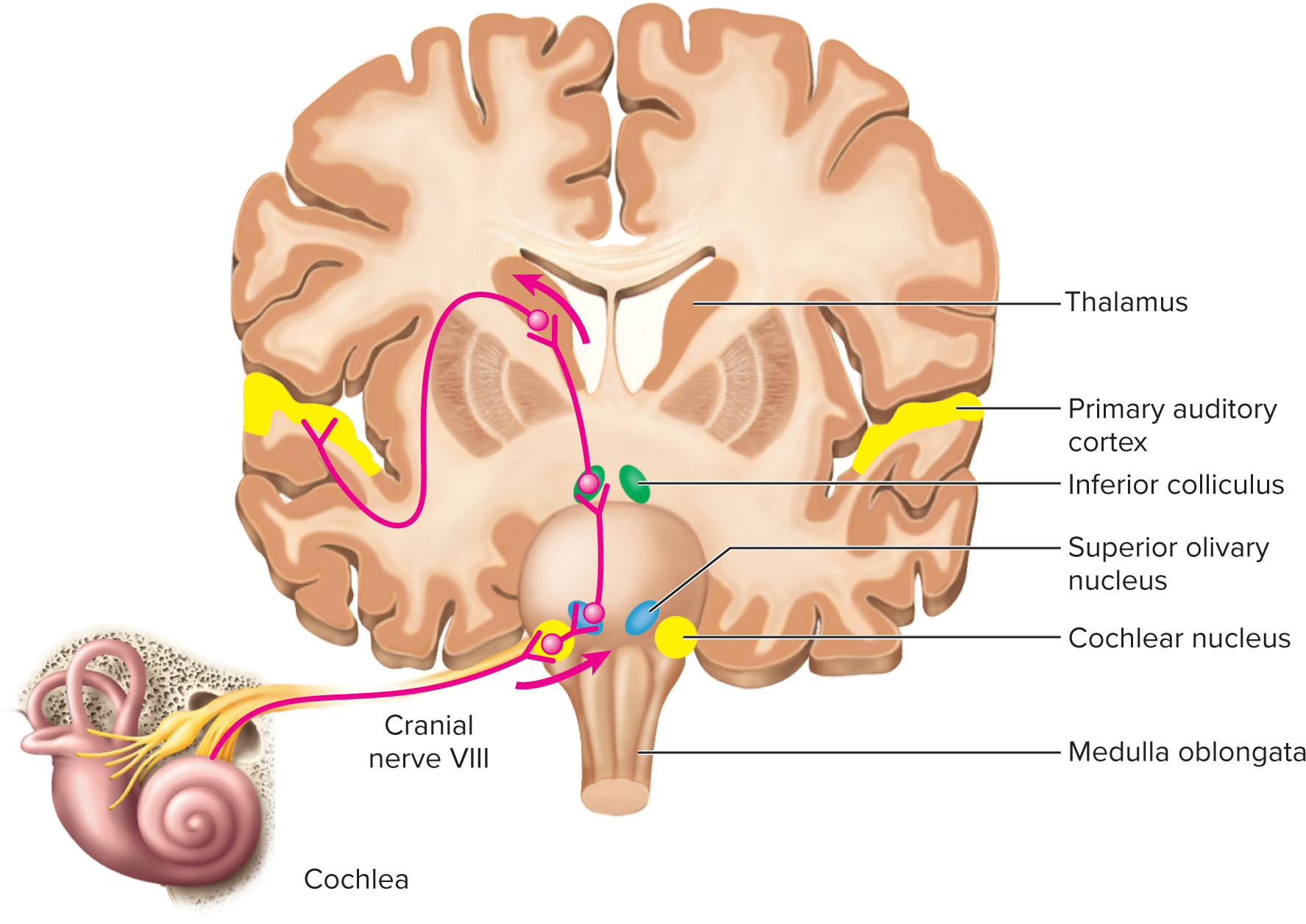
Auditory projection pathway:
First-order neurons:
Originate from spiral ganglion neurons (synapse with hair cells).
Transmit via cochlear nerve (CN VIII) to ipsilateral cochlear nuclei (medulla).
Second-order neurons:
Decussate (cross) and ascend to superior olivary nucleus (pons).
Superior olivary nucleus: sound localization (timing/intensity differences), bilateral processing, protective pathways.
Continue to inferior colliculi (midbrain).
Inferior colliculi: sound localization, integrate auditory information for reflexes (e.g., startle).
Third-order neurons:
Relayed from inferior colliculi to medial geniculate nucleus (thalamus).
Project from thalamus to primary auditory cortex (temporal lobe) for conscious sound perception.
Some fibers go to midbrain for quick, subconscious auditory reflexes.
Deafness types:
Conductive deafness: results from conditions that impede the transmission of sound vibrations through the outer or middle ear (e.g., otitis media, otosclerosis, perforated eardrum, excessive cerumen). Treatment often involves hearing aids or surgical correction.
Sensorineural deafness (nerve deafness): results from damage to the inner ear (hair cells) or the neural auditory pathway (cochlear nerve or brain centers). This is often irreversible and can be caused by prolonged exposure to loud noise, genetic factors, aging (presbycusis), or certain diseases. Cochlear implants can aid some cases of severe sensorineural loss.
16.4d The Physiology of Equilibrium
Equilibrium: the sense of body orientation, movement, and balance. It relies on the vestibular apparatus within the inner ear.
Vestibular apparatus: consists of three semicircular ducts (anterior, posterior, lateral) and two chambers: the anterior saccule and the posterior utricle. Both are part of the membranous labyrinth and are filled with endolymph.
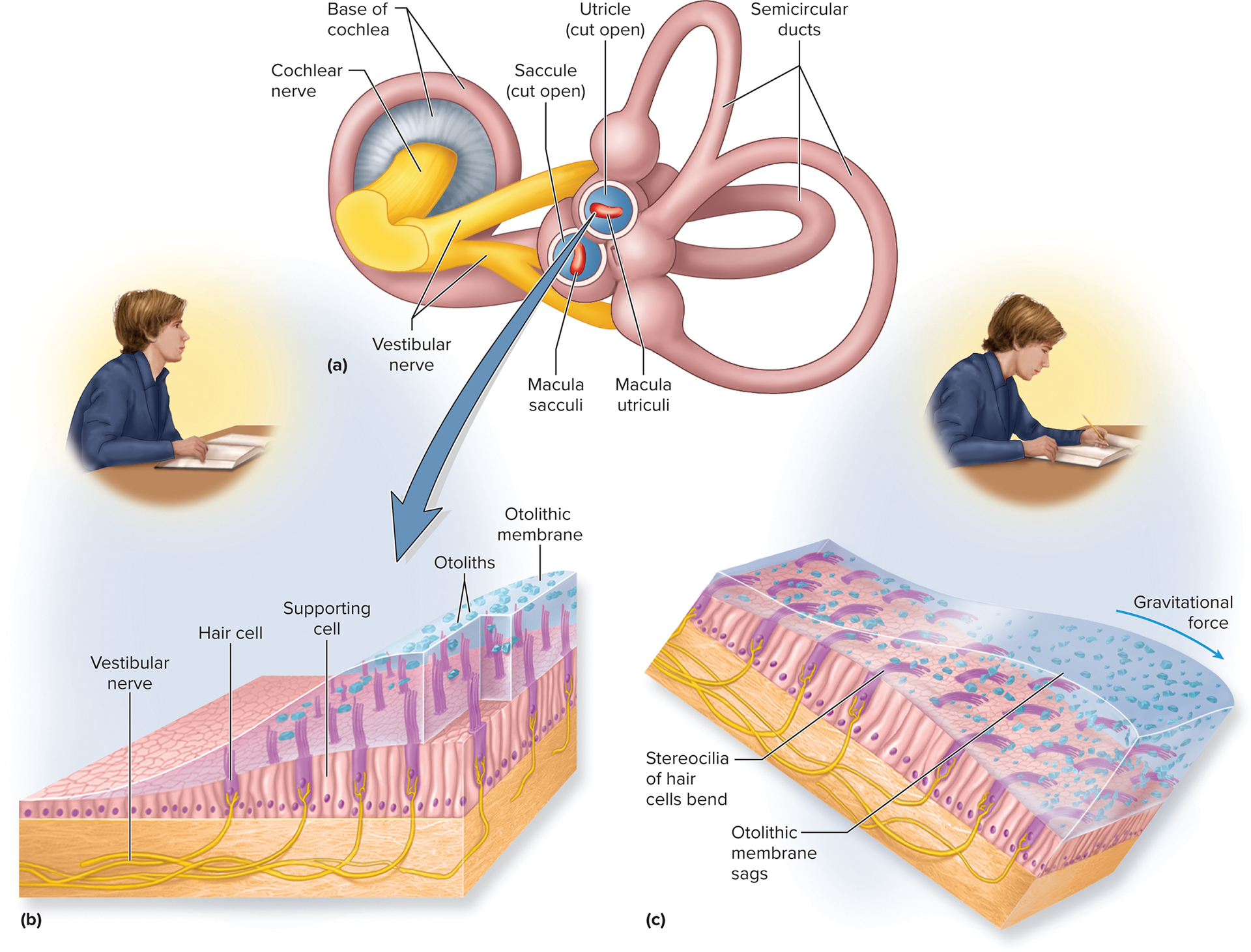
Static equilibrium (orientation): the perception of the orientation of the head when the body is stationary (e.g., tilting the head). This is detected by the maculae located within the saccule and utricle.
Static equilibrium (orientation):
Perception of head orientation when stationary.
Detected by Maculae: patches of hair cells and supporting cells in the saccule and utricle.
Stereocilia embedded in otolithic membrane (gelatinous layer with otoliths).
Head tilts/linear acceleration cause membrane to shift, bending hair cells.
Utricle's macula: sensitive to horizontal acceleration and head tilts.
Saccule's macula: sensitive to vertical acceleration.
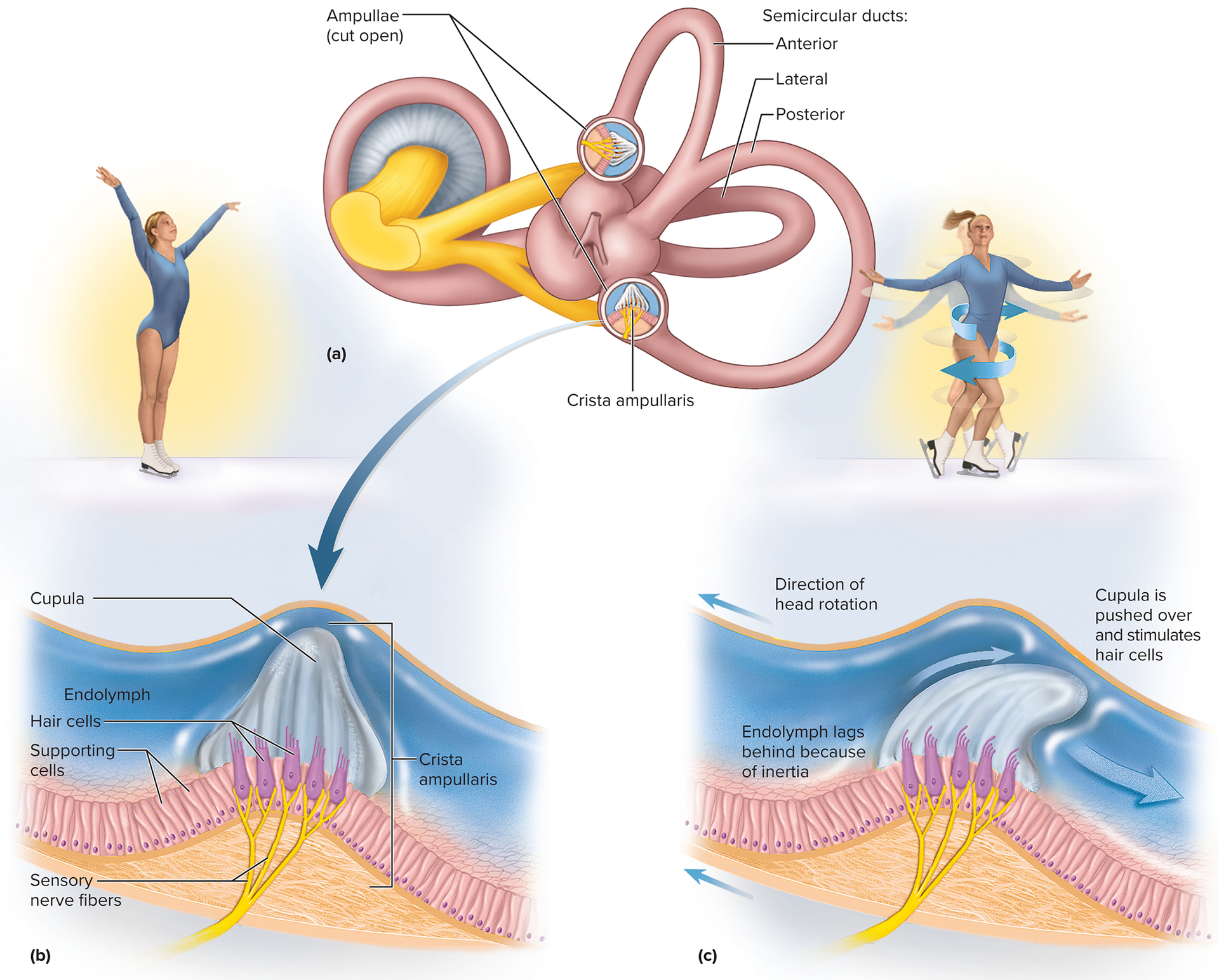
Dynamic equilibrium (movement perception):
Perception of rotational body movements.
Detected by Crista ampullaris: a ridge of hair cells and supporting cells in the ampullae of semicircular ducts.
Stereocilia embedded in a gelatinous cap called the cupula, extending into the endolymph.
During head rotation, endolymph inertia lags, pushing against and bending the cupula.
This stimulates hair cells, signaling direction and speed of rotation.
Three semicircular ducts detect rotation in all three dimensions.
Dynamic equilibrium (movement perception): the perception of motion or acceleration of the entire body, specifically rotational movements. This is detected by the crista ampullaris located in the ampullae (swelling at the base) of the semicircular ducts.
Crista ampullaris: a ridge of hair cells and supporting cells. Their stereocilia are embedded in a gelatinous cap called the cupula, which extends into the endolymph of the semicircular duct.
During head rotation, the inertia of the endolymph causes it to lag behind, pushing against and bending the cupula. This bending stimulates the hair cells, sending signals about the direction and speed of rotation.
Each of the three semicircular ducts is oriented in a different plane, allowing detection of rotation in all three dimensions.
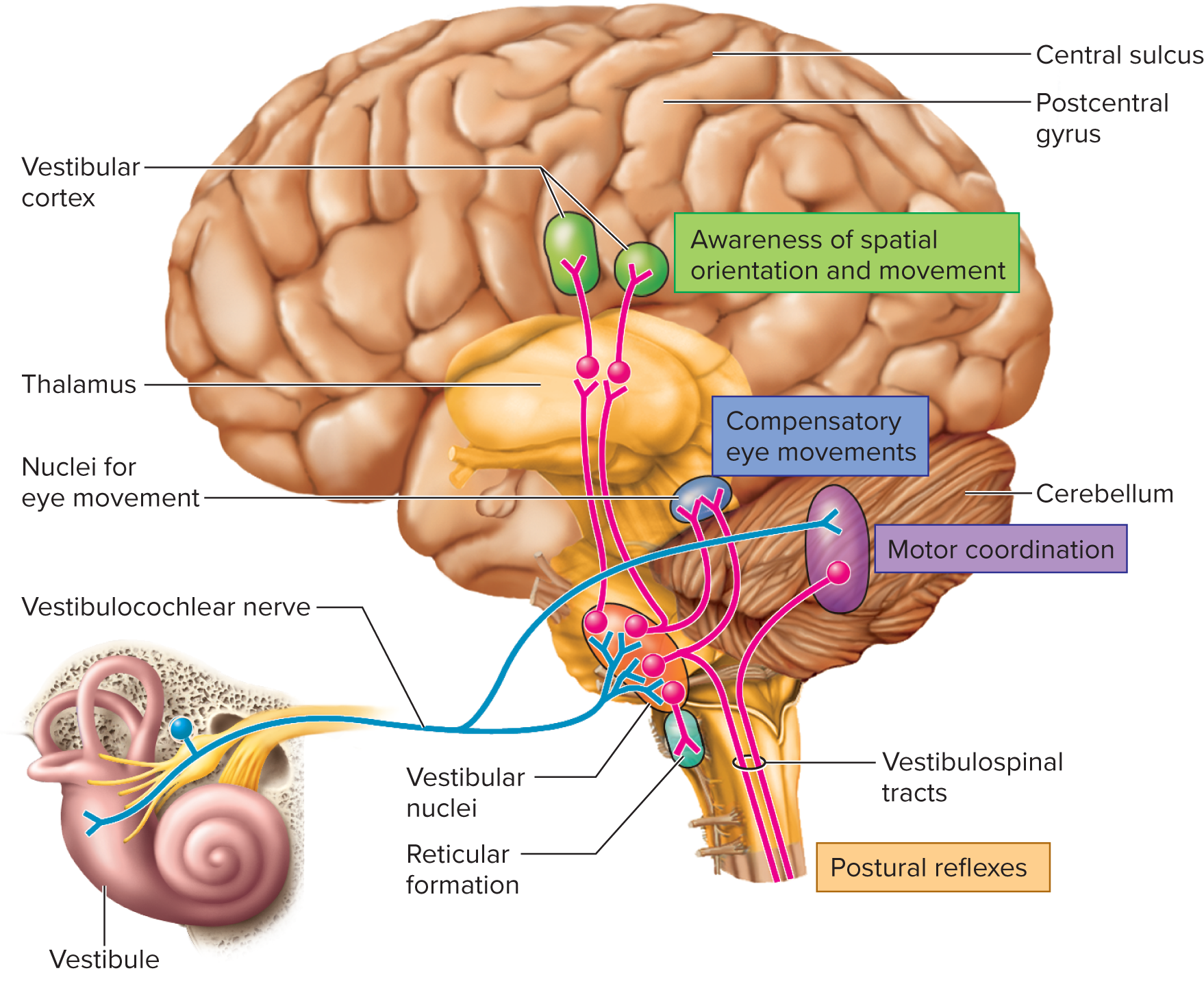
Projections: Information from the vestibular apparatus is carried by the vestibular nerve (a branch of CN VIII) to the vestibular nuclei located on the pons/medulla. These nuclei integrate vestibular information with visual and proprioceptive input.
Outputs from the vestibular nuclei travel to:
Cerebellum: for maintaining balance and coordinating posture and movement.
Reticular formation: for controlling respiration and cardiovascular function in response to changes in body position.
Spinal cord (via vestibulospinal tracts): for postural reflexes that adjust muscle tone to maintain balance.
Thalamus: for relay to the cerebral cortex (e.g., insula) for conscious awareness of body position and movement.
Cranial nerves III (oculomotor), IV (trochlear), and VI (abducens): to coordinate eye movements with head movements (vestibulo-ocular reflex), ensuring stable vision during head motion.
16.5 Vision
16.5a Light and Vision
Vision: the perception of objects based on the light they emit or reflect.
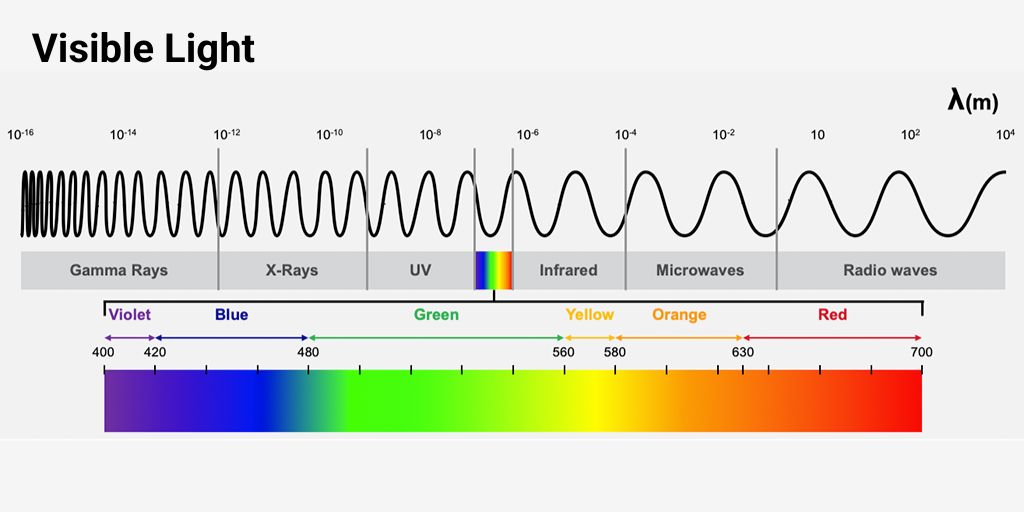
Light: visible electromagnetic radiation, which ranges from approximately 400 (violet) to 700nm (red) in wavelength for human vision.
For light to generate a nerve signal, it must cause a photochemical reaction in the photoreceptor cells of the retina.
Ultraviolet (UV) radiation: wavelengths below 400{ nm} ; invisible to humans. While some animals can see UV, in humans, it can damage macromolecules within the eye, especially the lens and retina.
Infrared (IR) radiation: wavelengths above 700{ nm} ; invisible to humans. The energy of IR photons is not sufficient to cause photochemical reactions in the retina but can warm tissues, including the eye.
16.5b Accessory Structures of the Orbit
The orbital region contains several structures that protect and aid the function of the eye:
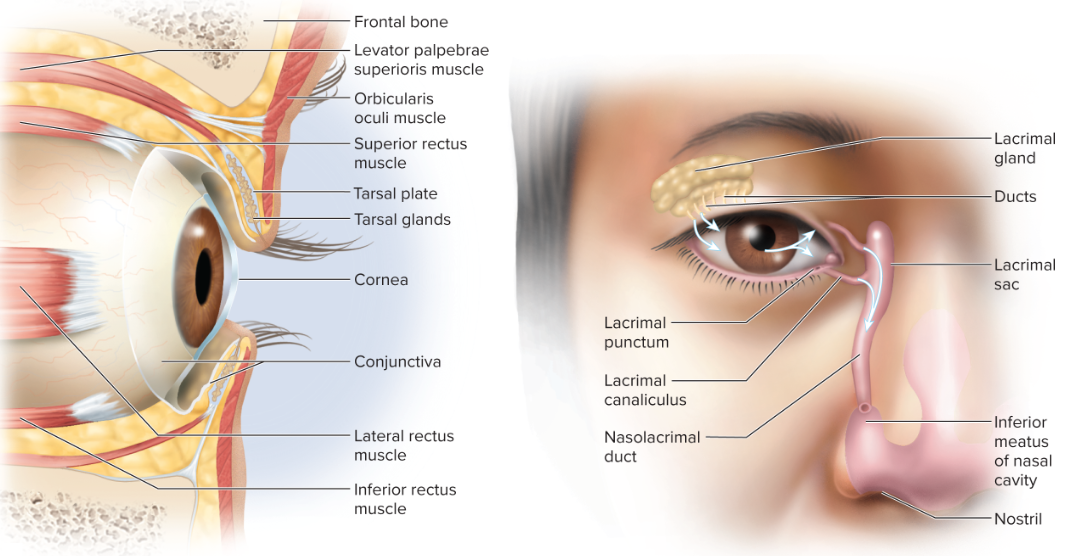
Eyebrows: shade the eyes from glare and prevent sweat from running into the eyes.
Eyelids (palpebrae): protect the eye from foreign objects, spread tears, and block light during sleep. They consist of:
Orbicularis oculi muscle: closes the eyelids.
Tarsal plate: a plate of dense connective tissue that gives the eyelids their shape and stiffness. It contains tarsal glands that secrete oil to prevent the eyelids from sticking together.
Eyelashes: guard hairs that help keep debris out of the eye.
Conjunctiva: a transparent mucous membrane that lines the inner surface of the eyelids (palpebral conjunctiva) and the anterior surface of the eyeball (bulbar conjunctiva), except for the cornea.
It lubricates the eye with a mucous film, is richly innervated (sensitive), and highly vascular (providing a blood supply for a portion of the sclera).
Lacrimal apparatus: responsible for tear production and drainage.
Lacrimal gland: located in the superolateral orbit, it produces tears (lacrimal fluid).
Lacrimal ducts: tears wash, nourish (with O2 nutrients), and protect (with lysozyme, an antibacterial enzyme) the eye.
They drain via the lacrimal punctum (small pores on the medial eyelid) → lacrimal canaliculi → lacrimal sac → nasolacrimal duct → nasal cavity.
Orbital fat: cushions and protects the eyeball within the bony orbit, allowing for smooth eye movements.
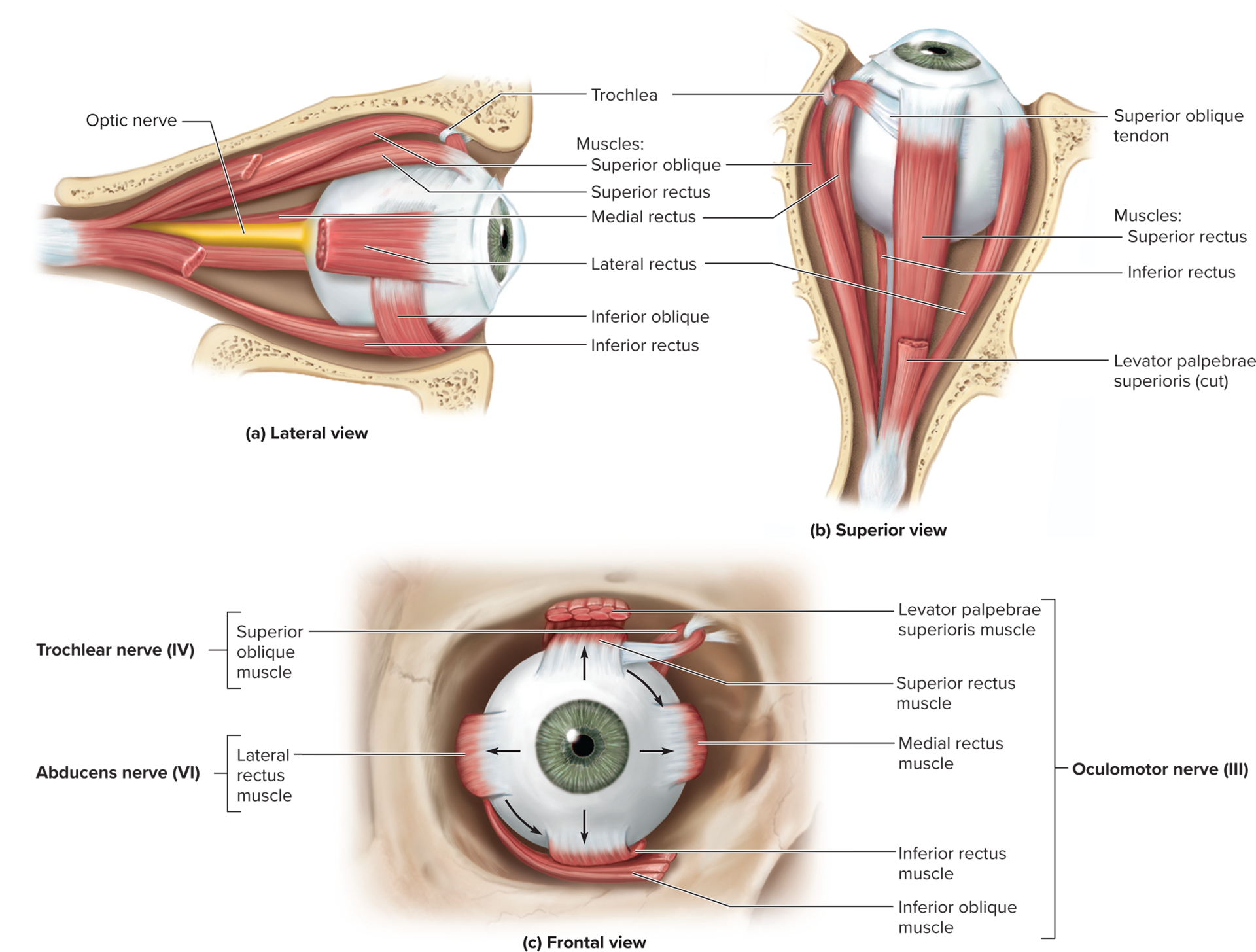
accessory structures of the orbit:
• Extrinsic eye muscles attach to exterior surface of eye
• Superior, inferior, medial, and lateral rectus
• Superior and inferior oblique
• Superior oblique tendon passes through trochlea
• Innervated by cranial nerves: CN IV innervates superior oblique, CN VI innervates lateral rectus, CN III innervates other four extrinsic muscles
• Movements:
• Superior, inferior, medial, and lateral rectus muscles move the eye up,
down, medially, and laterally (respectively)
• Superior and inferior obliques turn the “twelve o’clock pole” of each eye
toward or away from the nose; they also produce slight elevations and
depressions of the eye
16.5c Anatomy of the Eye: Tunics and external components
The eyeball is composed of three concentric tunics (layers):
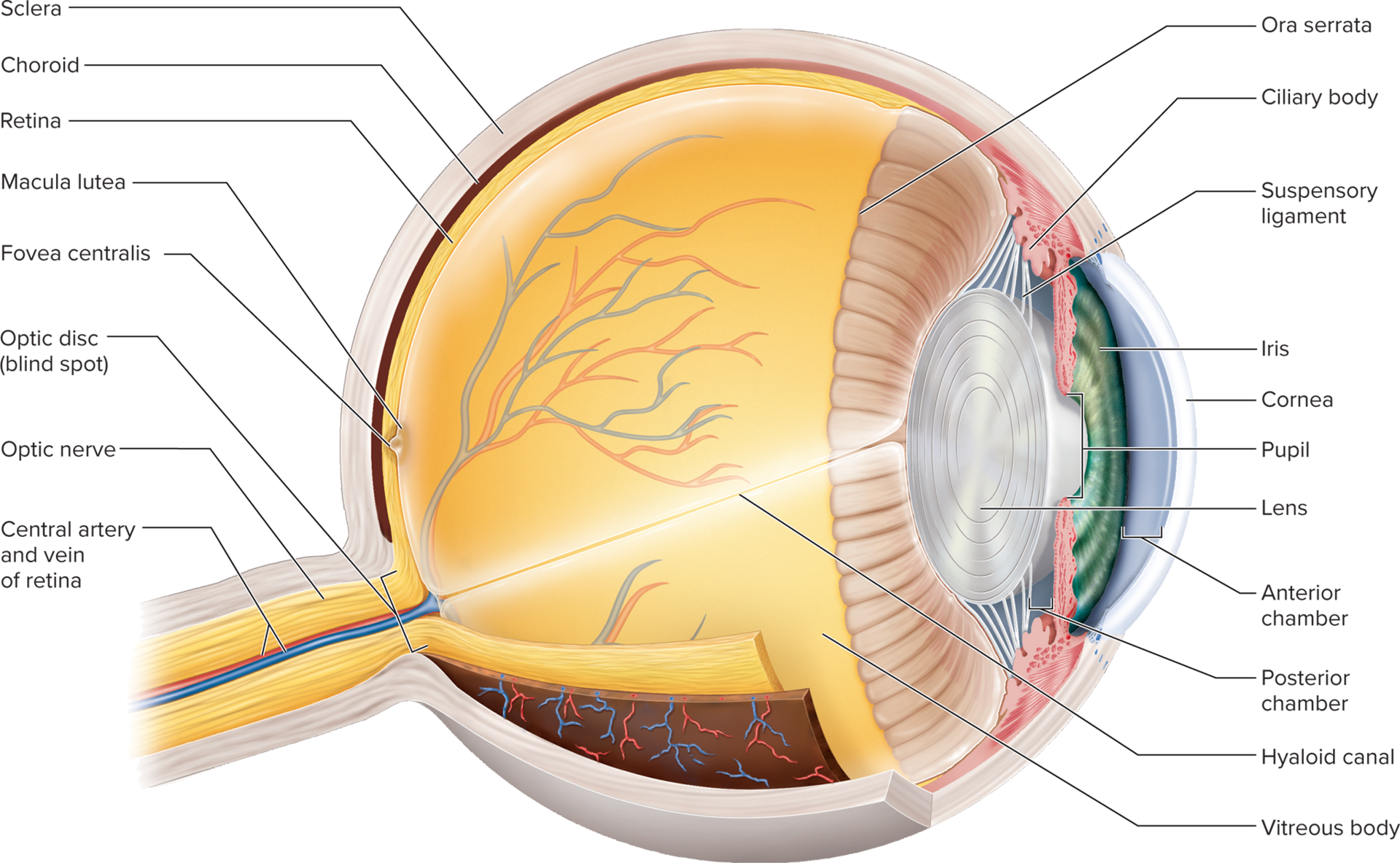
Fibrous layer (outermost): provides structural support and protection.
Sclera: the opaque, white, fibrous posterior portion of the eye; composed of dense collagen fibers.
It is tough and protective.
Cornea: the transparent, anterior portion of the fibrous layer.
It is avascular and its transparency and curved shape are critical for light refraction.
Vascular layer (uvea) (middle layer): rich in blood vessels and pigment.
Choroid: a highly vascular, dark brown pigmented layer posterior to the ciliary body.
It absorbs stray light to prevent reflection within the eye and provides nourishment to the retina.
Ciliary body: a muscular ring that encircles the lens.
It secretes aqueous humor and contains ciliary muscles which control the shape of the lens via suspensory ligaments.
Iris: the adjustable, colored diaphragm that controls the diameter of the pupil,
regulating the amount of light entering the eye.
Its color is due to melanin content.
Inner layer (innermost layer):
Retina: the light-sensitive layer at the back of the eye, containing photoreceptor cells and neurons that convert light into nerve signals.
It extends anteriorly to the ora serrata.
Beginning of the optic nerve: where retinal ganglion cell axons converge and exit the back of the eye.
Ora serrata: the irregularly scalloped anterior edge of the retina, marking the transition from the light-sensitive retina to the non-light-sensitive ciliary body.
16.5c Anatomy of the Eye (continued): external ocular structures
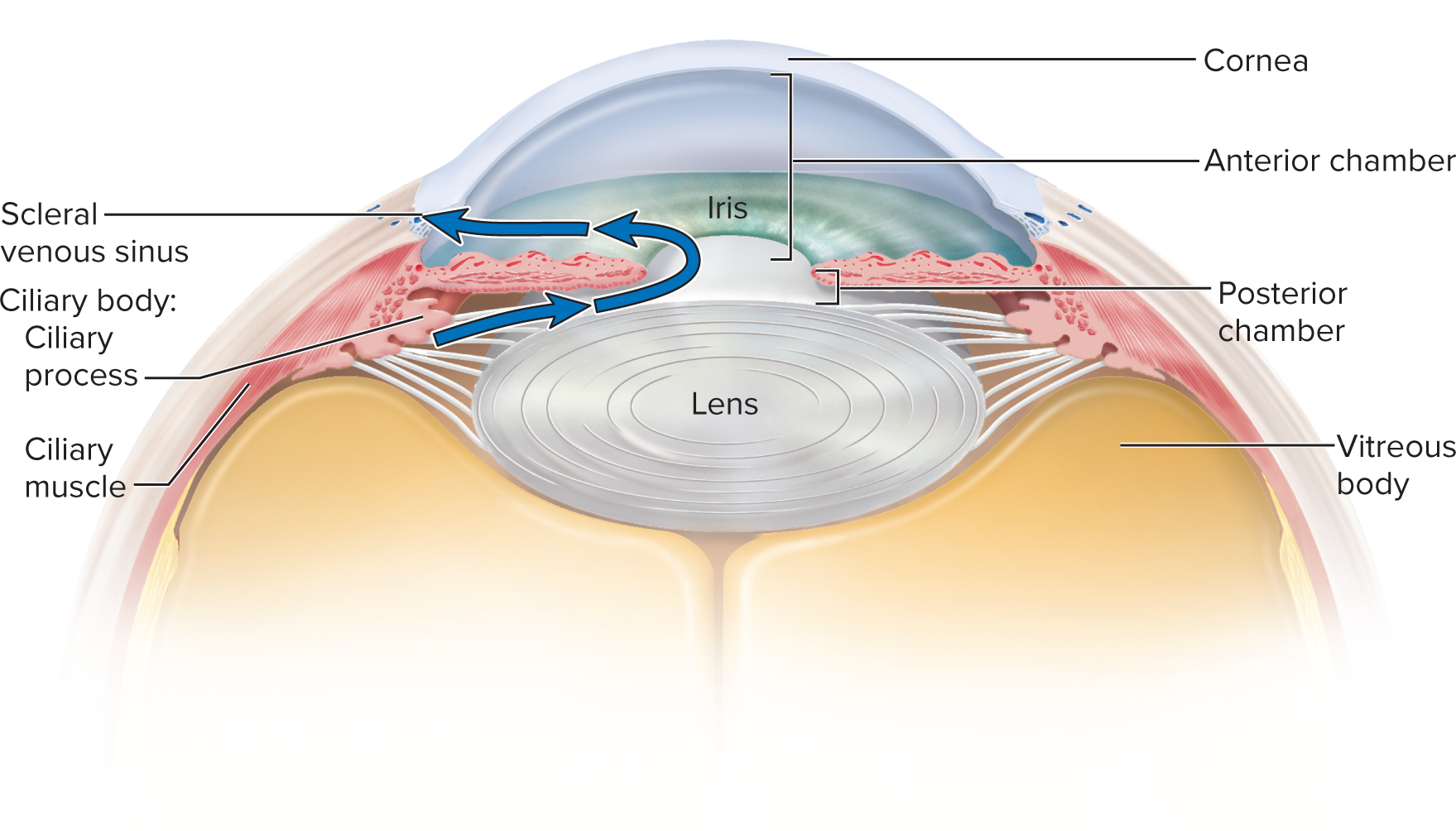
Visual pathway for aqueous humor: Aqueous humor is produced by the ciliary body and flows from the posterior chamber (between the iris and lens) through the pupil into the anterior chamber (between the cornea and iris).
It is then reabsorbed into the bloodstream via the scleral venous sinus (canal of Schlemm), a circular channel in the limbus (junction of cornea and sclera).
This constant production and drainage maintain intraocular pressure.
16.5c The optical components
These transparent elements admit, refract, and focus light onto the retina:
Cornea: Primary refractive component (2/32/3 of power); avascular, transparent, vital for clear vision.
Aqueous humor: Clear fluid in anterior/posterior chambers, nourishes cornea/lens, maintains intraocular pressure.
Lens: Biconvex structure behind iris; adjusts shape (accommodation) via suspensory ligaments to fine-tune focus. consists of laminated protein
Vitreous body (vitreous humor): Jelly-like substance filling posterior cavity; maintains intraocular pressure, holds retina
the choroid, and is largely static throughout life.
Hyaloid canal: an embryonic remnant within the vitreous body, typically no longer patent in adults.
16.4e The Lens of the Eye (SEM)
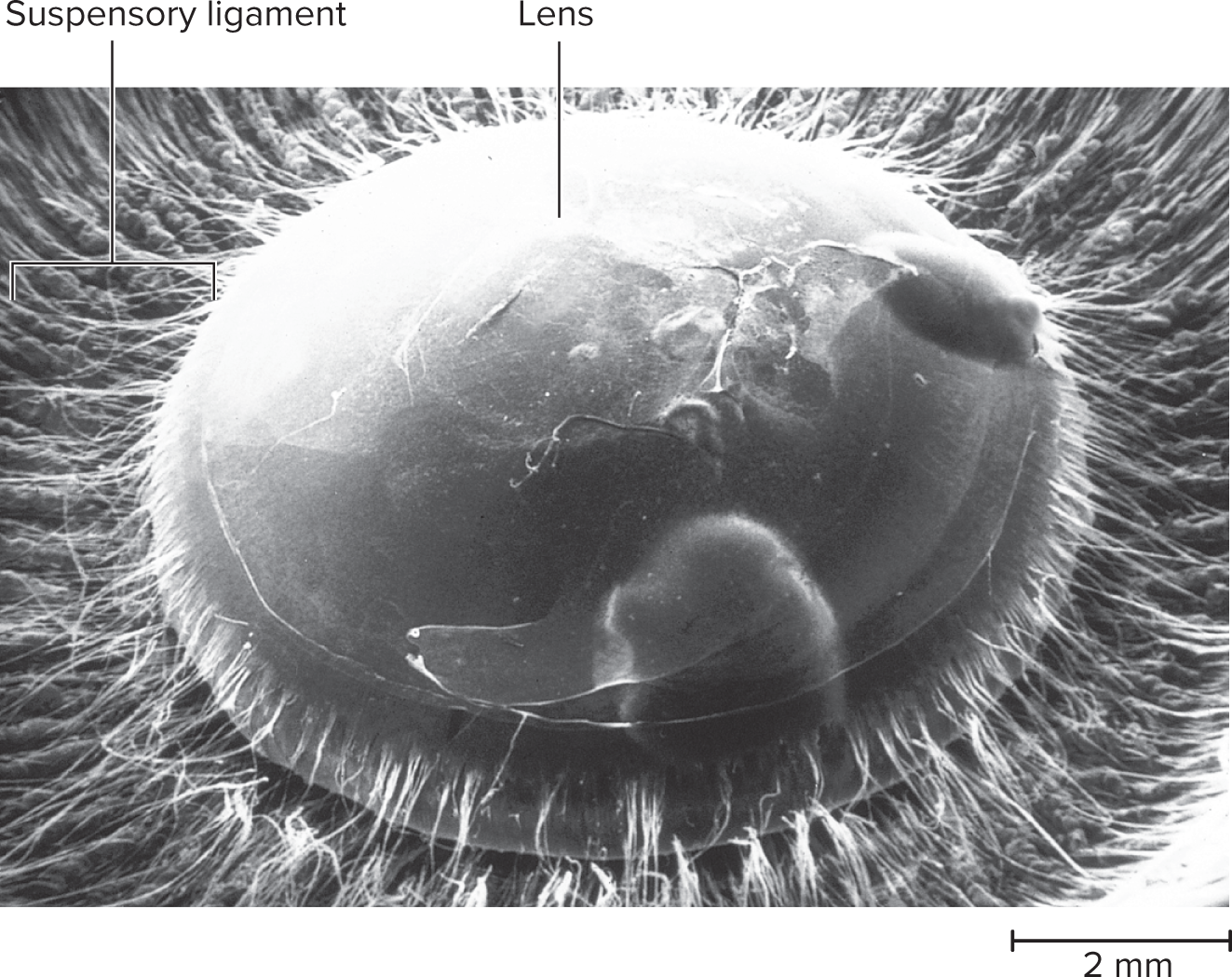
Lens: A transparent, avascular structure made of lens fibers, suspended by suspensory ligaments from the ciliary body.
Lens Shape & Vision:
Ciliary muscle contracts: releases ligament tension, allowing the lens to bulge (convex) for near vision.
Ciliary muscle relaxes: increases ligament tension, flattening the lens for distant vision.
16.4e Production and Reabsorption of Aqueous Humor
Aqueous humor is secreted by ciliary processes into the posterior chamber, flows through the pupil to the anterior chamber, and drains into the scleral venous sinus.
This flow maintains intraocular pressure; obstruction can cause its increase.
16.5d Formation of an Image
Light enters the eye, passes through optical components, and forms an inverted, real image on the retina.
Pupil Control: The iris regulates pupil size.
Pupillary Constrictor (Miosis): Parasympathetic (CN III) input contracts circular muscles, constricting the pupil in bright light or for near focus.
Pupillary Dilator (Mydriasis): Sympathetic input contracts myoepithelial cells, dilating the pupil in dim light or during excitement.
Photopupillary Reflex: A consensual reflex where both pupils constrict in response to bright light, protecting the retina.
16.5d Refraction and the Near Response
Refraction: The bending of light rays as they pass through different transparent media. The cornea provides most (2/3) of the eye's refractive power, with the lens fine-tuning the focus.
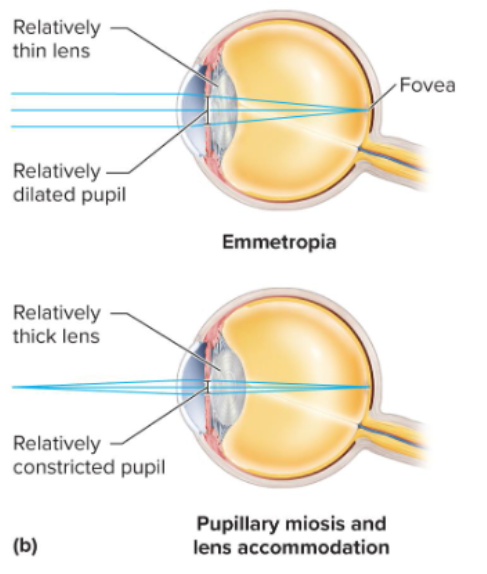
Emmetropia: eye is relaxed and focused on object more than 20 ft away
Light rays coming from that object are essentially parallel
Rays focused on retina without effort
Near Response: adjustment to close range vision on objects closer than 20 \text{ feet}.
Near Response Components:
Convergence: Eyes turn inward (medially) to align their visual axes on the near object.
Pupillary Constriction (Miosis): Pupils narrow to sharpen the image and increase depth of focus.
Lens Accommodation: change in the curvature of the lense
ciliary muscle contracts, suspensory ligaments slacken, and lens takes more convex (thicker) shape
Light refracted more strongly and focused onto retina
Near Point of Vision: The closest object that can be clearly focused with maximum accommodation; this distance typically lengthens with age (presbyopia) due to lens elasticity loss.
Lens shape changes with tension: When the ciliary muscle contracts, it releases tension on the suspensory ligaments, allowing the elastic lens to bulge and become more convex, increasing its refractive power for near vision. When the ciliary muscle relaxes, tension in the suspensory ligaments increases, flattening the lens for distant vision.
16.4e Production and Reabsorption of Aqueous Humor
(Details depicted in Fig. 16.27; mechanism of production/reabsorption not enumerated here.)
Aqueous humor is continually secreted into the posterior chamber by the ciliary processes, flows through the pupil into the anterior chamber, and drains into the scleral venous sinus (canal of Schlemm) in the limbus. Any obstruction to this drainage can lead to increased intraocular pressure.
16.5d Formation of an Image
The visual process begins as light enters the eye, passes through the optical components, and focuses on the retina, where it forms an inverted and real image.
Pupil size is controlled by the iris through two sets of contractile elements:
Pupillary constrictor (sphincter pupillae): composed of circular smooth muscles near the pupil. Parasympathetic innervation (via oculomotor nerve CN III) causes contraction, constricting the pupil (miosis) in bright light or for near focus.
Pupillary dilator (dilator pupillae): composed of spokelike myoepithelial cells. Sympathetic innervation causes contraction, dilating the pupil (mydriasis) in dim light or during emotional excitement.
Photopupillary reflex: a consensual reflex where both pupils constrict automatically in response to sudden bright light entering one or both eyes, protecting the retina from overstimulation.
16.5e Sensory Transduction in the Retina
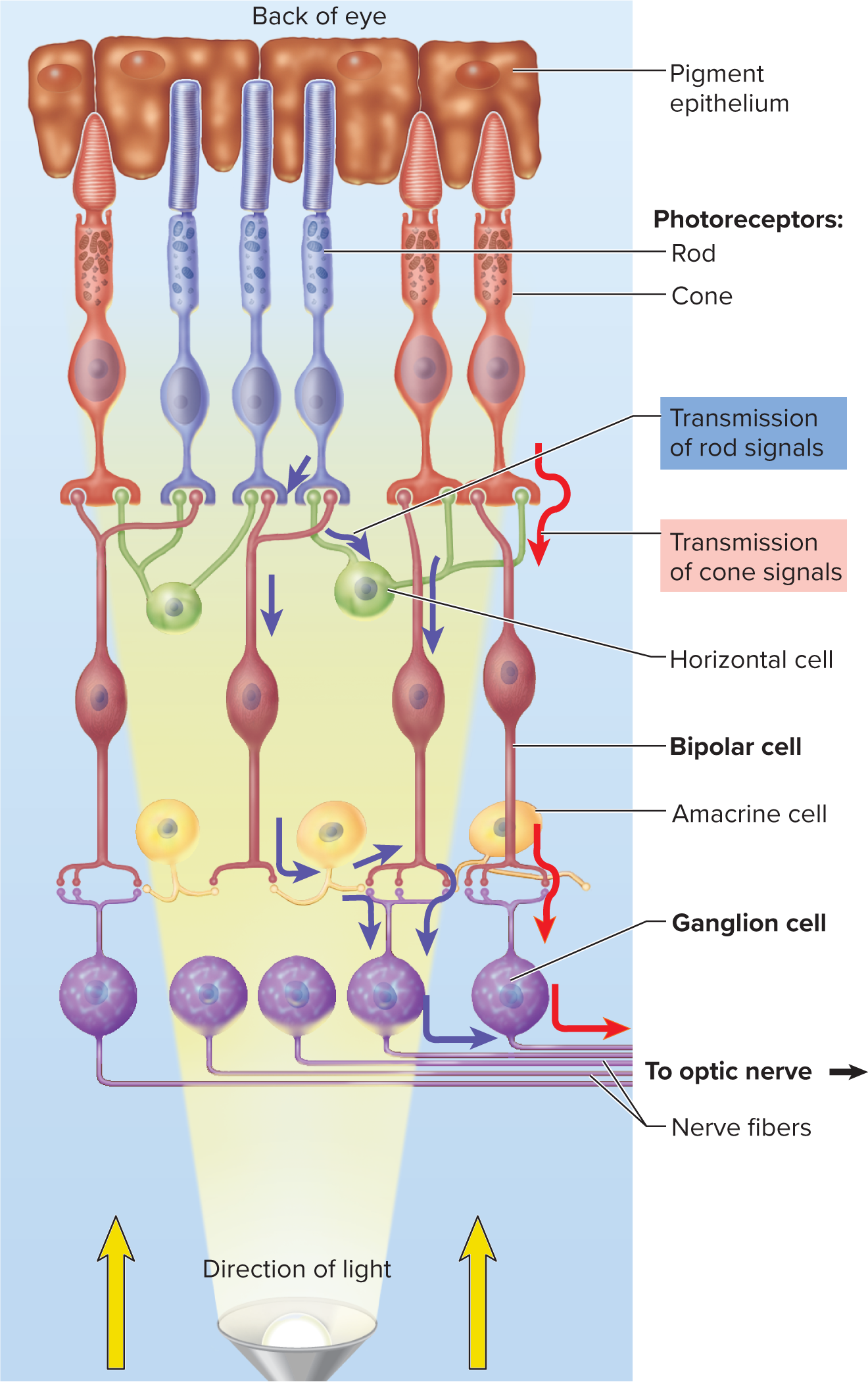
Retina layout: Innermost layer converting light to nerve signals. It consists of two main parts:
Pigmented layer (outer):most posteriror part of retina
absorbs stray light, prevents reflection, phagocytizes old photoreceptor discs, and stores vitamin A.
Neural components (inner): layers of cells responsible for signal processing and transmission.
Photoreceptor cells (rods and cones): transduce light energy into electrical signals.
Bipolar cells: receive input from photoreceptors, transmit to ganglion cells; can be "on-center" or "off-center."
Ganglion cells: axons form the optic nerve (CN II) to transmit signals to the brain.
Intrinsically photosensitive retinal ganglion cells (ipRGCs) containing melanopsin regulate pupil diameter and circadian rhythms.
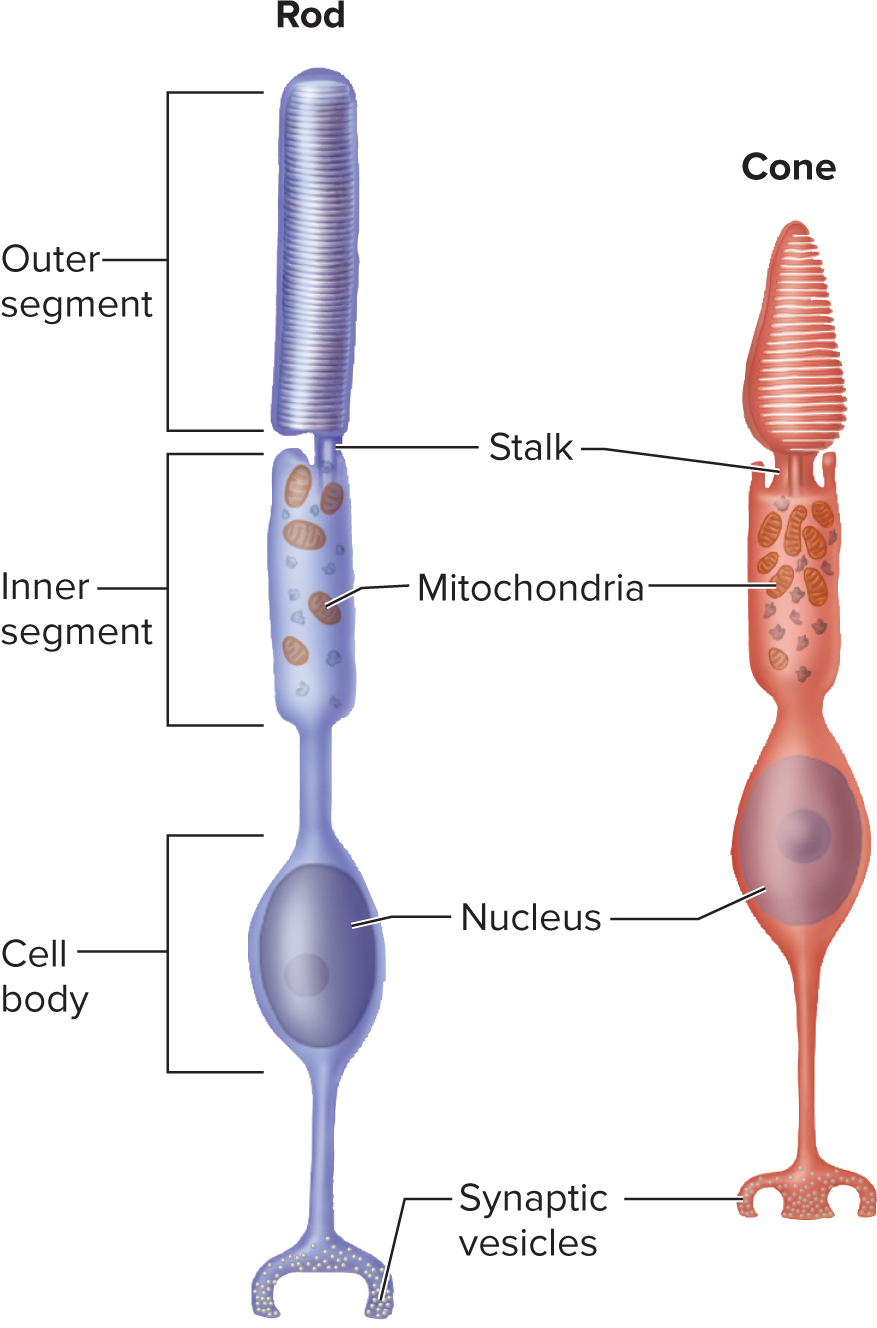
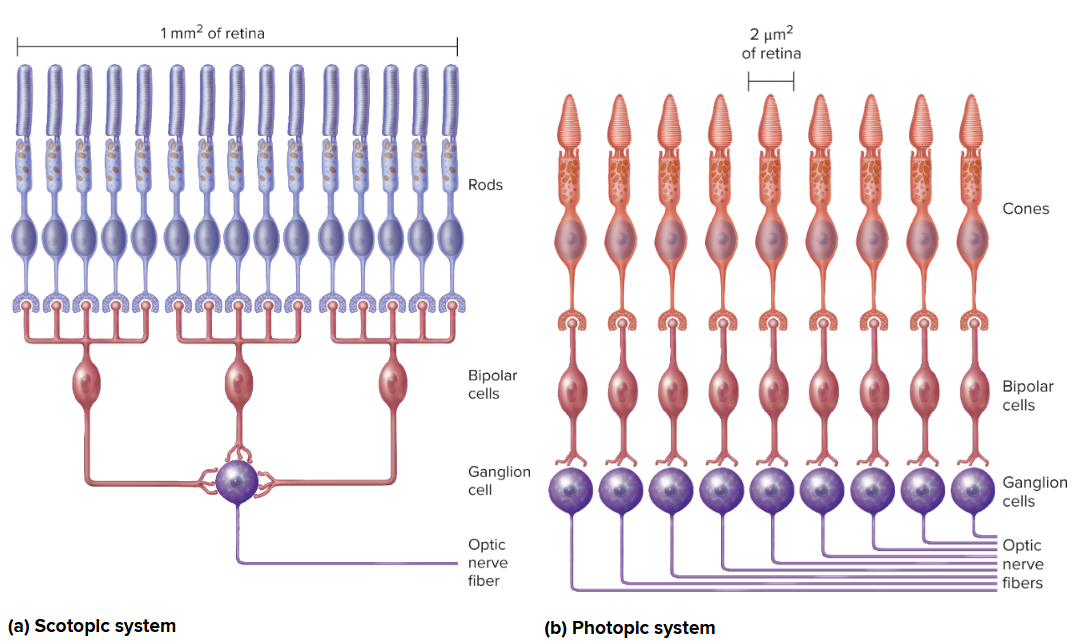
Rods: Highly sensitive, responsible for night (scotopic) vision and grayscale images.
More numerous and concentrated in the peripheral retina, they contain rhodopsin. High convergence leads to low spatial resolution.
Cones: Less sensitive, responsible for day (photopic) and color (trichromatic) vision, providing high acuity.
Concentrated in the fovea, they contain photopsin.
Photoreceptor disc renewal: New discs are continuously added at the basal end of the outer segment, pushing older discs to the tip, where they are shed and phagocytized by pigmented epithelium cells.
**Retinal circuitry:
Horizontal and amacrine cells: Retinal interneurons that enhance contrast (edge detection), amplify signals, detect motion, and respond to changes in light intensity.
Convergence: Many photoreceptors (especially rods) converge onto fewer bipolar and ganglion cells, increasing sensitivity to dim light but reducing resolution (common in periphery).
Low convergence (fovea): In the fovea, few cones converge per ganglion cell, enabling high visual acuity.
Visual pigments:
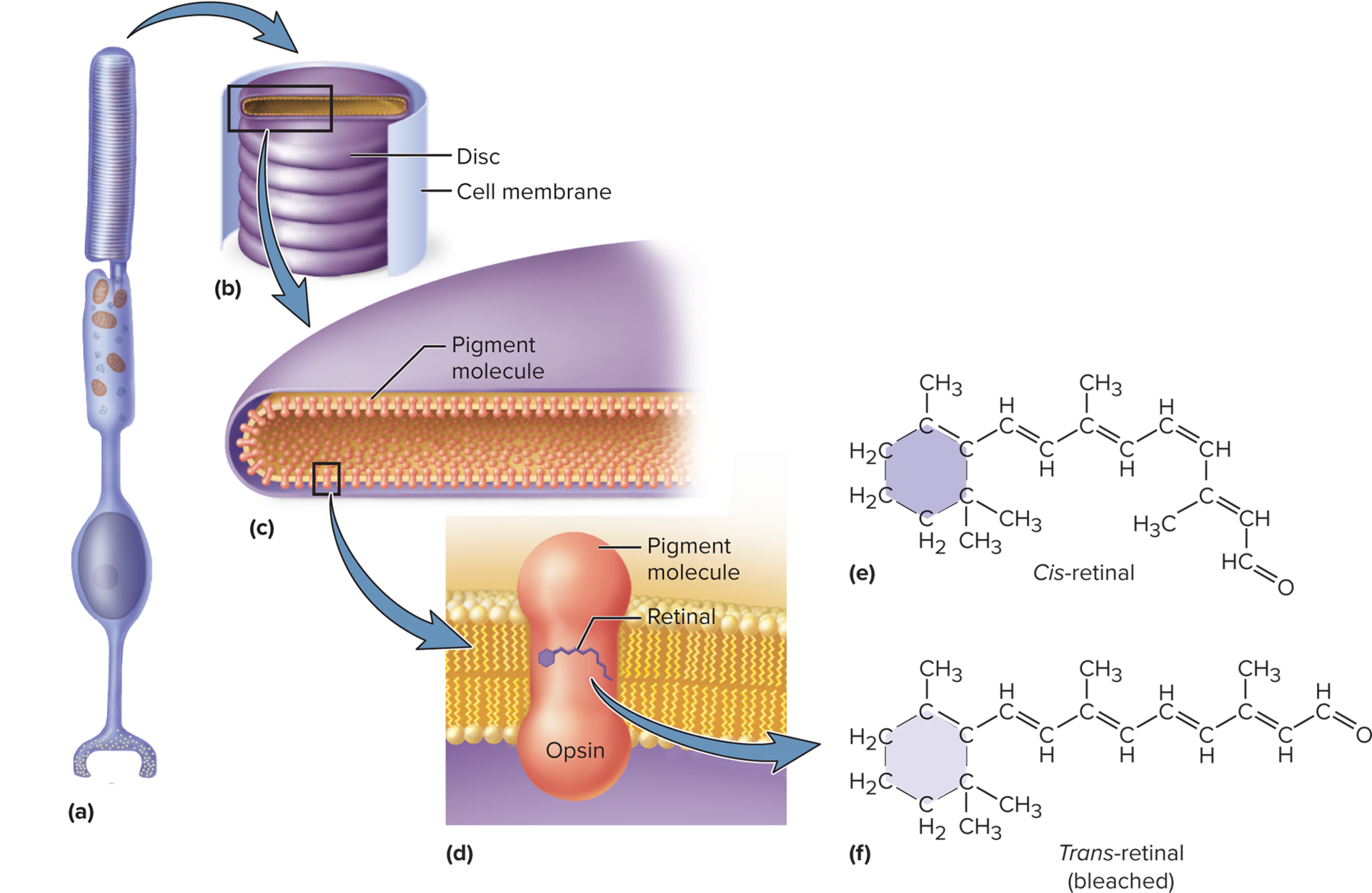
Rods: Contain rhodopsin, composed of opsin and 11-cis-retinal.
Rhodopsin displays maximal absorbance around 500{ nm} (green light).
Dietary vitamin A deficiency impairs rhodopsin synthesis, causing night blindness.
Cones: Contain photopsin, composed of retinal combined with one of three different types of opsin proteins, allowing for color vision based on varying peak absorbances.
Cone peak wavelengths: Three types of cones, each maximally sensitive to a different range:
S (short-wavelength) cones: peak at approx 420nm (blue).
M (medium-wavelength) cones: peak at approx531 nm (green).
L (long-wavelength) cones: peak at approx558{ nm} (red-yellow).
Visual pigments: regeneration and bleaching:
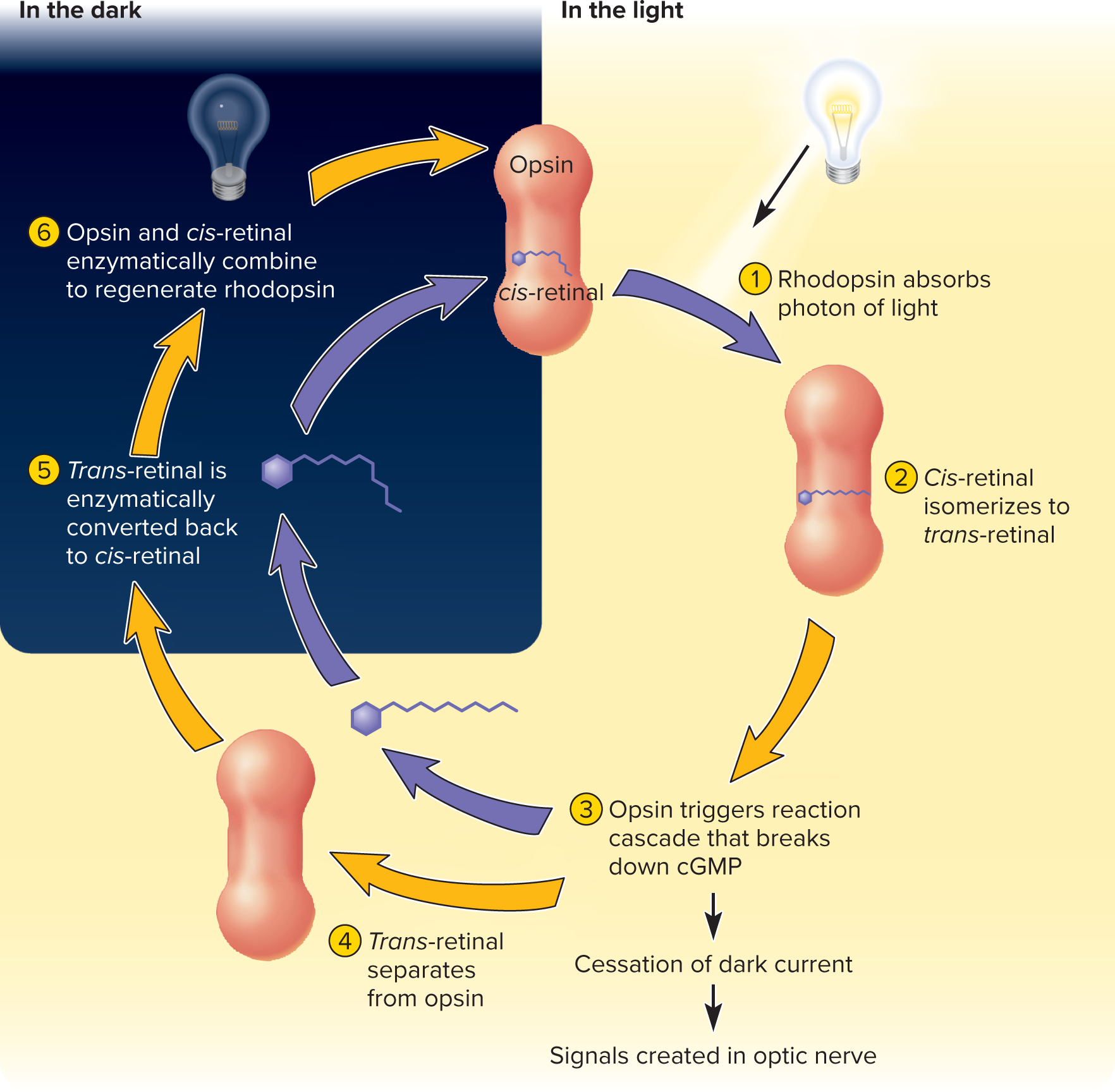
In darkness: Retinal is in its 11-cis-retinal form, bound to opsin, forming rhodopsin or photopsin.
With light exposure: 11-cis-retinal absorbs a photon, straightens to all-trans-retinal, and dissociates from opsin, causing bleaching (the pigment becomes colorless).
Regeneration: All-trans-retinal converts back to 11-cis-retinal and rebinds with opsin. Rhodopsin regeneration is relatively slow (50\% in = 50min, full in \approx20-30{ min}); 50% approx 9- 10 minutes) due to differences in the sensitivity and adaptation of the cones compared to the rods.
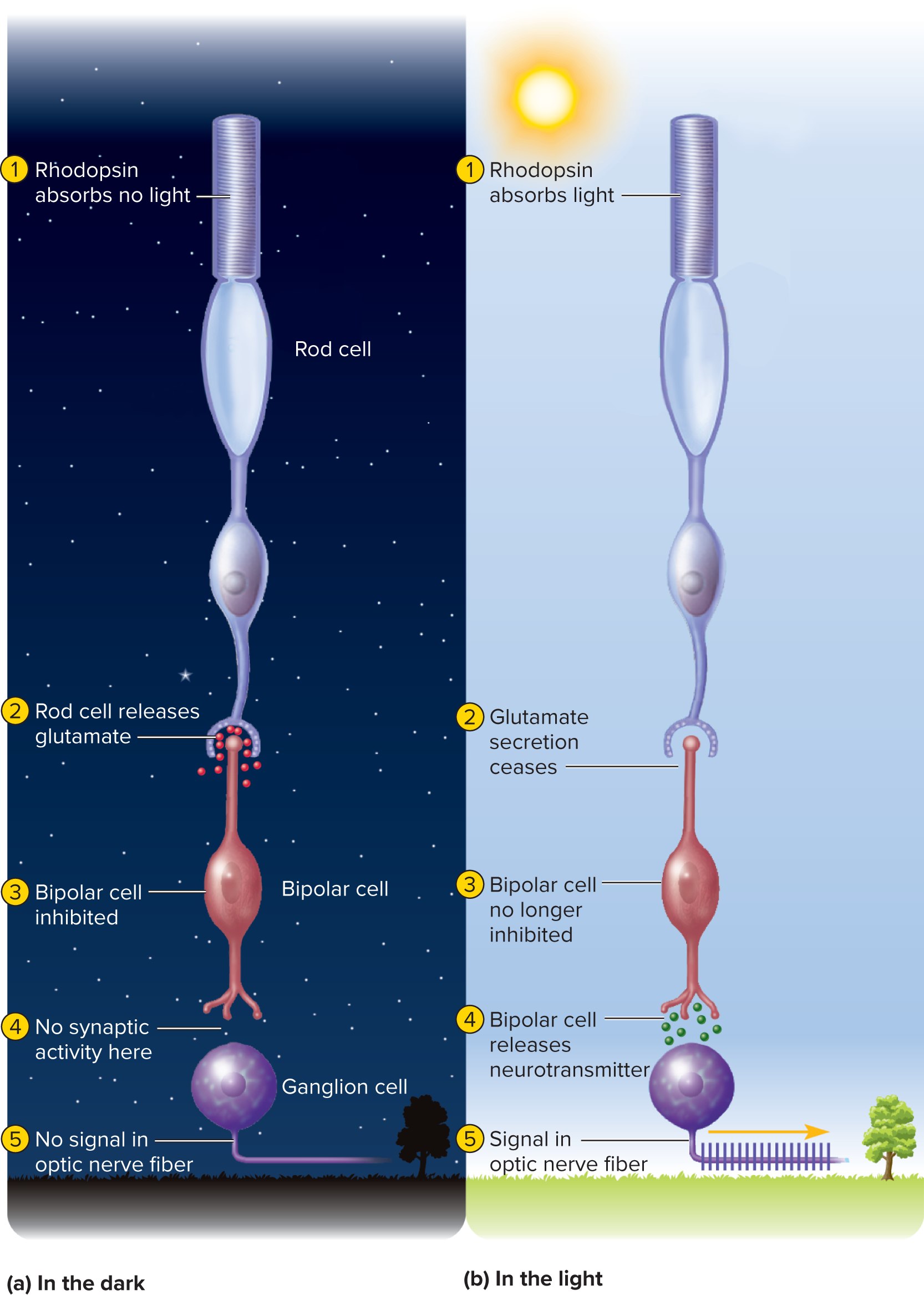
**Signal generation in retina:
In darkness:** Rod cells are depolarized by a "dark current" (open cGMP-gated Na+ and Ca2+ channels) and continuously release glutamate at synapses with bipolar cells.
In light: Activated transducin and phosphodiesterase break down cGMP, causing cGMP-gated channels to close. This hyperpolarizes the rod cell and decreases glutamate release.
Bipolar cell response: "On-center" bipolar cells are inhibited by glutamate (in dark) and excited (depolarized) by its decrease (in light). "Off-center" bipolar cells are excited by glutamate (in dark) and inhibited by its decrease (in light).
Ganglion cells: Bipolar cells transmit graded potentials to ganglion cells. Sufficient stimulation triggers action potentials in ganglion cells, which are the only signals transmitted through the optic nerve to the brain for visual perception.
16.5e Retina histology
Rods: Highly sensitive photoreceptors for night (scotopic) and grayscale vision. Most common in the peripheral retina, they contain rhodopsin. Many rods converge to process light, increasing sensitivity but lowering detail.
Cones: Less sensitive photoreceptors for day (photopic) and color (trichromatic) vision, providing sharp detail (acuity). Concentrated in the fovea, they contain photopsin.
Photoreceptor discs: Both rods and cones continuously grow new discs at their base. Older discs are shed from the tips and are then eaten (phagocytized) by surrounding pigmented cells.
16.5e Sensory Transduction in the Retina
Photoreceptors (Rods & Cones):
Rods: Highly sensitive for night (scotopic) and grayscale vision; mainly peripheral. Contain rhodopsin. Many rods converge, boosting sensitivity but reducing detail.
Cones: Lower sensitivity but provide high acuity and color vision for daytime; concentrated in the fovea. Contain photopsin.
Photoreceptor discs are continuously renewed (new discs added, old ones shed and phagocytized).
Retinal Circuitry:
Horizontal and amacrine cells are interneurons that enhance contrast, amplify signals, and detect motion.
High convergence in peripheral retina (many rods to few ganglion cells) increases dim light sensitivity but lowers resolution.
Low convergence in the fovea (few cones per ganglion cell) enables high visual acuity.
Visual Pigments:
Rhodopsin (Rods): Composed of opsin and 11-cis-retinal, peaks at 500 \text{ nm}. Vitamin A deficiency causes night blindness.
Photopsin (Cones): Retinal + three types of opsin proteins, enabling color vision:
S-cones: peak at \approx 420 \text{ nm} (blue).
M-cones: peak at \approx 531 \text{ nm} (green).
L-cones: peak at \approx 558 \text{ nm} (red-yellow
16.5e Visual pigments: regeneration and bleaching
In darkness, 11-cis-retinal binds to opsin. Light bleaches pigments by converting 11-cis-retinal to all-trans-retinal, which detaches. Regeneration reforms 11-cis-retinal and rebinds; rhodopsin (rods) regenerates slowly (\approx [20-30] \text{ min}), while photopsin (cones) regenerates rapidly (50\% in \approx 90 \text{ sec}).
16.5e Signal generation in retina
In darkness, depolarized rod cells release glutamate (due to open cGMP-gated Na+ and Ca2+ channels via a "dark current").
In light, activated transducin/phosphodiesterase break down cGMP, closing channels, hyperpolarizing the rod cell, and decreasing glutamate release.
Bipolar cells respond to glutamate changes: "On-center" cells are inhibited by glutamate (dark) and excited by its decrease (light); "Off-center" cells are excited by glutamate (dark) and inhibited by its decrease (light).
Bipolar cells transmit graded potentials to ganglion cells, which generate action potentials that travel via the optic nerve to the brain (thalamus, then visual cortex) for conscious perception.
16.5f Light and Dark Adaptation
Light adaptation: occurs when moving from a dark environment to a bright one. This is a rapid process involving:
Immediate pupil constriction via the photopupillary reflex to reduce light entry.
Rapid bleaching of large amounts of rhodopsin and photopsin, causing a temporary reduction in sensitivity and initial blinding glarea.
Color vision and acuity are reduced for the first 5–10 minutes while the retina adjusts and pigment regeneration begins.
Cone vision takes over as rods become saturated and nonfunctional in bright light.
Dark adaptation: occurs when moving from a bright environment to a dark one. This is a slower process involving:
Pupil dilation to allow more light to enter.
Gradual regeneration of rhodopsin in rods, which were bleached in bright light. This regeneration significantly increases the sensitivity of the rods.
Night vision begins to improve in ~1–2 minutes, but maximum sensitivity (rod vision) is reached only after ~20–30 minutes, as rhodopsin levels are fully restored.
16.5g The Dual Visual System
Duplicity theory of vision: states that a single photoreceptor system cannot provide both high sensitivity (for dim light vision) and high resolution (for sharp, detailed vision and color). Instead, the retina has two distinct systems:
Rods: specialized for scotopic (night) vision. They have high sensitivity to light (can be activated by a single photon) due to the large amount of rhodopsin and high convergence onto ganglion cells. However, this convergence leads to low spatial resolution and no color discrimination.
Cones: specialized for photopic (day) vision and color vision. They have lower sensitivity (require more light) but provide high resolution (due to low convergence, especially in the fovea) and trichromatic color perception.
The brain integrates information from both systems depending on ambient light conditions.
16.5h Color Vision
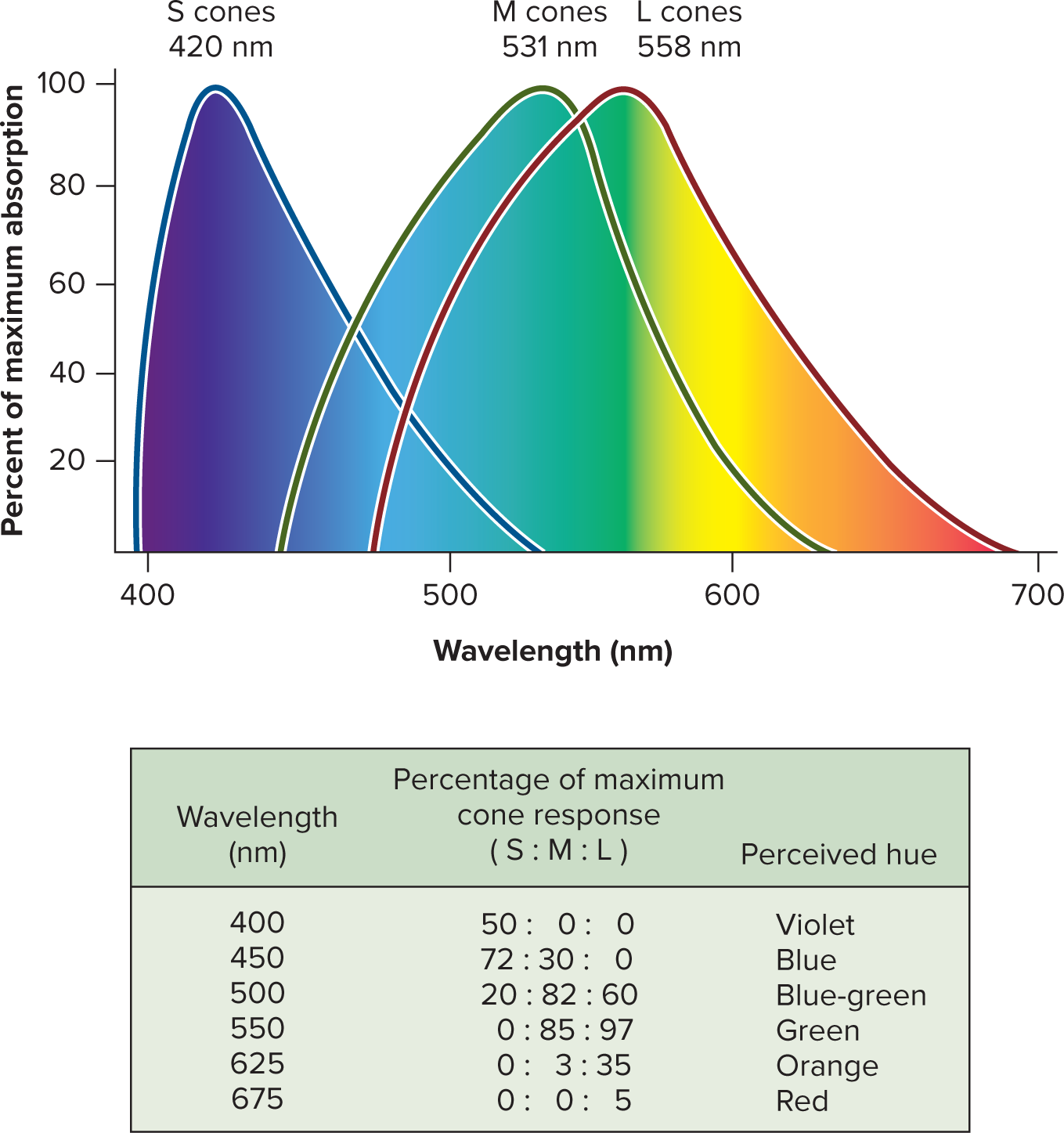
Color vision is based on the trichromatic (Young-Helmholtz) theory, which posits that color perception arises from the differential stimulation of the three types of cones:
S (short-wavelength) cones: maximally sensitive to blue light (peak \approx 420 ext{ nm}).
M (medium-wavelength) cones: maximally sensitive to green light (peak \approx 531 ext{ nm}).
L (long-wavelength) cones: maximally sensitive to red-yellow light (peak \approx 558 ext{ nm}).
A given wavelength of light activates all three cone types to differing degrees. The brain then interprets the color based on the unique pattern and ratio of activation across these three cone types (e.g., green light stimulates M cones most, but also L cones and slightly S cones).
Genetic variations in the opsin genes can lead to common color vision deficiencies (e.g., red-green color blindness), which are typically X-linked and thus more prevalent in males.
16.5i Stereoscopic Vision
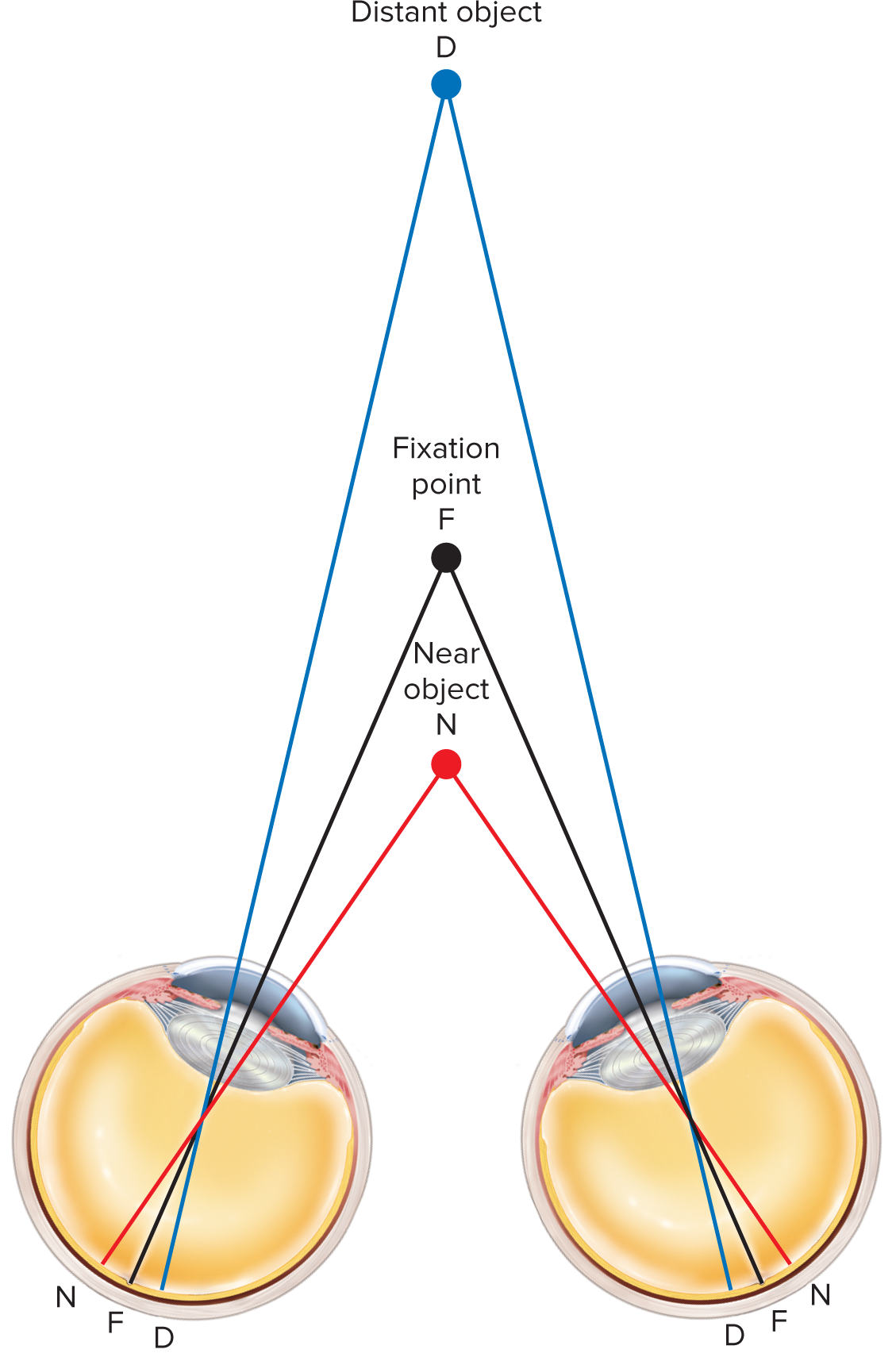
Stereopsis (stereoscopic vision): Depth perception and the ability to judge distances, which arises from having two eyes with overlapping visual fields (binocular vision). The brain combines the slightly different images received by each eye.
Fixation point: The specific point in space on which both eyes are focused. Objects at varying distances from the fixation point will project to slightly different (disparate) locations on each retina.
Objects within approximately 100 ext{ feet} (or 30 ext{ meters}) are viewed from slightly different angles by each eye. The brain uses these disparities to compute depth. For objects beyond this distance, light rays are nearly parallel, and depth perception relies more on monocular cues (e.g., relative size, linear perspective).
Panoramic vision (e.g., in prey animals with eyes on the sides of their heads) provides a wide field of view but lacks significant depth perception.
16.5j The Visual Projection Pathway
Retina: Bipolar cells (first-order) synapse with photoreceptors; retinal ganglion cells (second-order) generate action potentials.
Optic nerve (CN II): Formed by retinal ganglion cell axons exiting the eye.
Optic chiasm: Nasal (medial) retina fibers decussate (cross); temporal (lateral) fibers remain ipsilateral. This ensures each brain hemisphere processes the contralateral visual field.
Optic tracts: Extend from the chiasm.
passing laterally around the hypothalamus with most axons ending in lateral geniculate nucleus of thalamus
Lateral Geniculate Nucleus (LGN) of the thalamus: Main relay point for most optic tract axons, processing and filtering visual information.
Optic radiation: Third-order neurons from LGN project to the primary visual cortex.
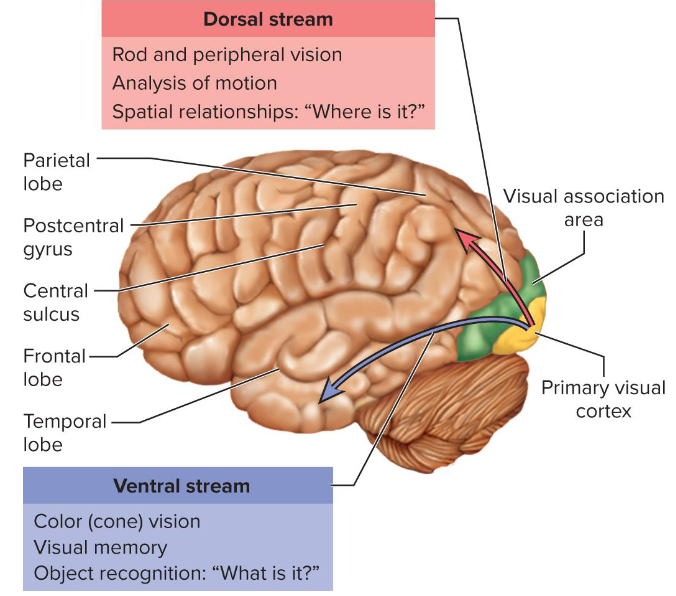
Primary visual cortex: In the occipital lobe, for conscious visual perception. Information splits into ventral ("what" - object identification) and dorsal ("where" - spatial/motion) streams.
few optic nerve fibers project to midbrain and terminate in superior colliculi and pretectal nuclei
superior colliculi controls visual reflexes of extrinsic eye muscles
pretectal nuclei are involved in photopupillary and accommodation reflexes
some processing begins in retina
Primary visual cortex is connected by association tracts to
visual association areas in parietal and temporal lobes which
process retinal data from occipital lobesVentral stream—pathway through lower temporal lobe;
concerned with color vision, object location, and visual
memory (recognized words, faces, identify things we see)Dorsal stream—pathway through upper parietal lobe;
concerned with recognizing locations, spatial relationships
of objects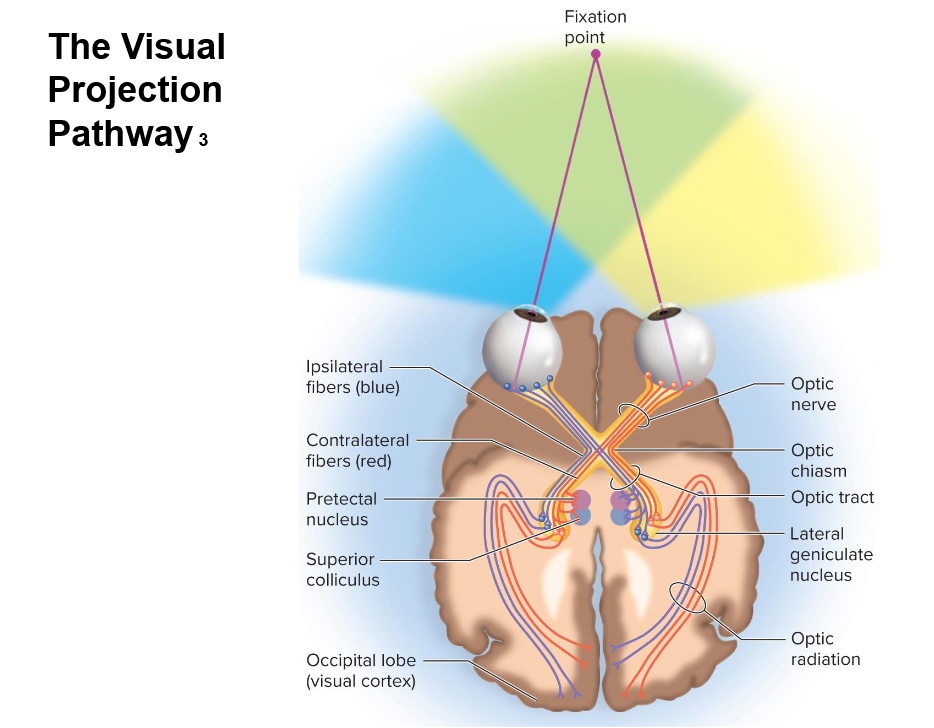
Midbrain projections: Some fibers bypass LGN to superior colliculi (eye movements/reflexes) and pretectal nuclei (pupil/accommodation reflexes).
Common Visual Defects:
Cataracts: Lens clouding leading to blurry vision; treated by lens replacement.
Glaucoma: Elevated intraocular pressure due to obstructed aqueous humor drainage; damages optic nerve, causing vision loss, often peripheral.
Macular Degeneration: Photoreceptor loss in macula/fovea, leading to central vision loss.
Diabetic Retinopathy: Diabetes-related damage to retinal blood vessels, causing vision loss.
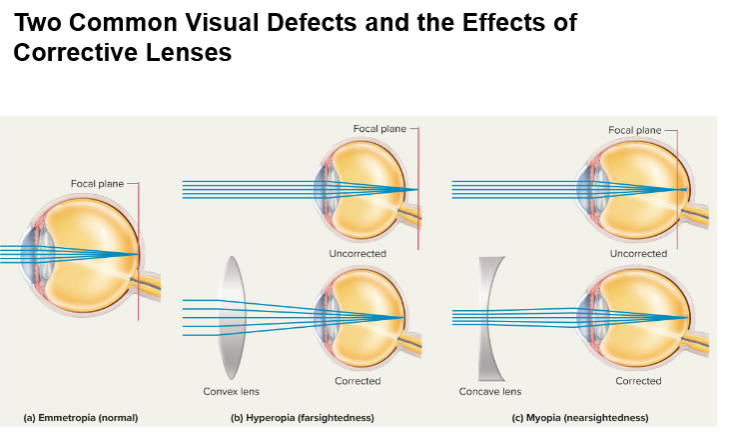
Myopia (Nearsightedness): Distant objects focus in front of retina (long eyeball/curved lens); corrected with concave lenses.
Hyperopia (Farsightedness): Distant objects focus behind retina (short eyeball/flat lens); corrected with convex lenses.
Astigmatism: Unequal corneal/lens curvature causing blurred vision; corrected with cylindrical lenses.
Retina and Optic Nerve Details:
Retina: Part of the brain, attached at optic disc and ora serrata; vitreous humor maintains its position.
Optic Disc (Blind Spot): Area where optic nerve exits; no photoreceptors.
Macula Lutea: Yellowish patch for high-resolution vision.
Fovea Centralis: Center of macula with highest cone concentration for peak visual acuity and color discrimination.
Fundus: Rear wall of the eye visible through ophthalmoscope.
Refractive/Defensive Aspects:
Aqueous Humor: Maintains intraocular pressure, nourishes avascular cornea/lens.
Photopupillary & Accommodation Reflexes: Coordinate pupil size and lens shape for precise light entry and focus.
Connections, Implications, and Additional Notes:
General Sensory Design: Stimulus detection by receptors, energy conversion to signals (transduction), neural transmission to CNS, conscious perception.
Sensory Adaptation: Phasic (fast) vs. Tonic (slow) adaptation filters constant stimuli.
Receptive Field/Convergence: Determine acuity and sensitivity (high convergence = high sensitivity, low resolution; low convergence = low sensitivity, high resolution).
Pain Modulation: Brain alters pain perception via descending pathways and endogenous opioids.
Dual Visual System: Rods for dim light/sensitivity; cones for bright light/resolution/color.
Somatic vs. Visceral Senses: Different neural circuits; referred pain from visceral-somatic convergence.
Eye Anatomy: Optical components (cornea, lens, humors) and dynamic focusing (accommodation) form sharp images.
Color Vision (Trichromatic Theory): Based on differential activation of S, M, L cones.
Sensory-Limbic Ties: Strong links between senses (especially olfaction) and memory/emotion.
Quantitative Details:
Hearing: Pitch 20-20,000 ext{ Hz}; loudness
60 ext{ dB} (conversation),
120-140 ext{ dB} (pain threshold).Pain Fiber Speeds: A-delta (12-30 ext{ m/s}), C-fibers (0.5-2 ext{ m/s}).
Cone Peak Sensitivities: S (\approx 420 ext{ nm} blue), M (\approx 531 ext{ nm} green), L (\approx 558 ext{ nm} red-yellow).
Retina and optic nerve details:
Retina is part of the brain, attached at optic disc (blind spot where optic nerve exits) and ora serrata, held by vitreous humor.
Macula lutea: yellowish patch for high-resolution vision; Fovea centralis: center of macula, highest cone concentration for peak acuity/color.
Fundus: rear wall of the eye, visible via ophthalmoscope.
Refractive/defensive aspects:
Aqueous humor maintains intraocular pressure, nourishes avascular cornea/lens.
Photopupillary and accommodation reflexes coordinate pupil size and lens shape for precise light entry and focus.
Connections, implications, and additional notes
General Sensory Design: Stimulus detection by specialized receptors, transduction into electrical signals, transmission to CNS, and conscious perception.
Sensory Adaptation: Phasic (fast) vs. Tonic (slow) adaptation filters constant stimuli.
Receptive Field/Convergence: Determine acuity and sensitivity (high convergence = high sensitivity, low resolution; low convergence = high resolution).
Pain Modulation: Brain alters pain perception via descending pathways and endogenous opioids.
Dual Visual System: Rods for dim light/sensitivity; cones for bright light/resolution/color, integrated by the brain.
Somatic vs. Visceral Senses: Differing neural circuits; referred pain due to visceral-somatic convergence in CNS.
Eye Anatomy: Optical components (cornea, lens, humors) and dynamic focusing (accommodation) form sharp images on the retina.
Color Vision (Trichromatic Theory): Relies on differential activation of S, M, L cones; genetic variations cause deficiencies.
Sensory-Limbic Ties: Strong links (especially olfaction) with memory and emotion.
Quantitative Details:
Hearing: Pitch 20-20,000 ext{ Hz}; loudness 60 ext{ dB} (conversation), 120-140 ext{ dB} (pain threshold).
Pain Fiber Speeds: A-delta (12-30 ext{ m/s}), C-fibers (0.5-2 ext{ m/s}).
Cone Peak Sensitivities: S (\approx 420 ext{ nm} blue), M (\approx 531 ext{ nm} green), L (\approx 558 ext{ nm} red-yellow).
Visual pigment absorption: rhodopsin peak at \approx 500 ext{ nm} (green light); cone photopsins have distinct maxima.
Refractive/defensive aspects
Aqueous humor production and reabsorption continuously maintains a stable intraocular pressure, which is essential for maintaining the eye's shape and ensuring proper retinal function.
Photopupillary and accommodation reflexes work synergistically to coordinate pupil size and lens shape, respectively, ensuring that the appropriate amount of light enters the eye and that images are precisely focused on the retina for clear vision at various distances and light levels.
Connections, implications, and additional notes
The senses share a general design: detection of a stimulus by specialized receptors, conversion of stimulus energy into electrical signals (transduction and receptor potential generation), transmission of these signals via hierarchical neural pathways to the CNS, and finally, conscious perception and interpretation by the brain.
Sensory adaptation (phasic vs tonic) is a fundamental general principle observed across various modalities. It underlies the brain’s ability to filter out constant, non-critical signals and to focus on novel or changing stimuli, which is crucial for survival and attention.
Receptive field size and neuronal convergence determine both sensory resolution (acuity) and sensitivity. High convergence (e.g., many rods converging on a single ganglion cell in the peripheral retina) increases sensitivity to dim light but reduces detail. Conversely, low convergence (e.g., foveal cones having almost a direct, private line connection to the brain) enhances resolution and acuity, albeit at the cost of sensitivity.
Pain modulation illustrates the brain’s remarkable capacity to alter sensory perception via descending inhibitory pathways and endogenous opioids. This inherent system has significant clinical relevance for understanding and developing strategies for analgesia and pain management.
The dual visual system (rods for night vision/sensitivity and cones for day vision/resolution/color) perfectly explains phenomena like our ability to see in both dim and bright light, perceive color, and judge depth. The brain further integrates information across specialized processing streams (e.g., the ventral stream for object identification and color, and the dorsal stream for spatial relationships and motion).
Receptors and neural circuits differ significantly for somatic (from body surface and musculoskeletal system) versus visceral (from internal organs) sensations. Referred pain is a classic example of CNS integration, where the brain misinterprets the origin of visceral pain signals due to the convergence of visceral and somatic afferents in the spinal cord.
The eye’s intricate optical components and dynamic focusing mechanisms (accommodation) exemplify how anatomy and physiology cooperate to form a coherent, sharp image on the retina. Disorders such as cataracts, glaucoma, macular degeneration, and diabetic retinopathy highlight the critical importance of maintaining ocular health for vision.
Color vision, governed by the trichromatic theory, depends on the differential activation of three distinct cone types (S, M, L). Genetic variations in the genes encoding cone photopsins are responsible for common color vision deficiencies (e.g., red-green color blindness), which exhibit characteristic sex-linked inheritance patterns.
The senses are deeply interconnected with memory and emotion. Olfactory inputs, in particular, have strong, unique ties to limbic structures (amygdala, hippocampus), explaining why smells can powerfully influence mood, trigger vivid memories, and affect behavior. Taste and visual cues also play significant roles in emotional responses and memory formation.
Mathematical/quantitative details to recall:
Pitch range for human hearing: f \text{ in } [20, \text{ }20{,}000] \text{ Hz}.
Loudness range: typical conversation is about 60 \text{ dB}; the threshold for pain is approximately 120 \text{–}140 \text{ dB}.
Nerve conduction speeds for pain fibers: v{\text{A-delta}} \text{ up to } [12,30] \text{ m/s}; \, v{\text{C}} \text{ about } [0.5,2] \text{ m/s}.
Cone peak sensitivities for color vision: S (short-wavelength) cone: maximum absorbance at \approx 420 \text{ nm} (blue); M (medium-wavelength) cone: maximum absorbance at \approx 531 \text{ nm} (green); L (long-wavelength) cone: maximum absorbance at \approx 558 \text{ nm} (red-yellow).
Visual pigment absorption: rhodopsin peak at \approx 500 \text{ nm} (green light); cone photopsins have distinct maxima corresponding to S/M/L categories.
Summary diagrams (to study from memory)
Receptors \rightarrow Transduction (receptor potential) \rightarrow Sensation \rightarrow Perception
Three-neuron somatosensory pathway: First-order (from receptor to CNS) \rightarrow Second-order (decussates, ascends to thalamus) \rightarrow Third-order (from thalamus to cortex)
Auditory pathway: Cochlea \rightarrow Cochlear nuclei (medulla) \rightarrow Superior olivary nucleus (pons) \rightarrow Inferior colliculus (midbrain) \rightarrow Thalamus (Medial Geniculate Nucleus) \rightarrow Primary auditory cortex (temporal lobe)
Visual pathway: Retina \rightarrow Optic nerve \rightarrow Optic Chiasm (hemidecussation) \rightarrow Optic tract \rightarrow LGN (Lateral Geniculate Nucleus of thalamus) \rightarrow Optic radiation \rightarrow Primary visual cortex (occipital lobe) with dorsal/ventral streams
Key terms to remember
Receptor potential, Sensation, Perception, Receptive field, Phasic vs Tonic, Modality, Proprioceptor, Exteroceptor, Interoceptor, Nociceptor, Mechanoreceptor, Chemoreceptor, Photoreceptor, Olfactory mucosa, Taste buds, Gustation, Olfaction, Meissner corpuscle, Pacinian corpuscle, Ruffini corpuscle, Merkel discs, Hair receptors, Basilar membrane, Organ of Corti, Endolymph, Perilymph, Rhodopsin, Photopsin, Cone types (S/M/L), Glomerulus, Odorant receptor, Afferent/efferent pathways, Vestibulo-ocular reflex, Macula, Otoliths, Cupula, Saccule, Utricle, Crista ampullaris, Otolithic membrane, Convergence, Accomodation, Myopia, Hyperopia, Astigmatism, Cataracts, Glaucoma, Macular degeneration, Diabetic retinopathy