Atomic Theory Full Notes
1.1 Ancient Understandings
Greek Concept of Matter
Democritus: Proposed that all matter is made of tiny, indivisible particles called Atomos.
Believed that if you kept cutting something in half, eventually, you'd reach a point where it couldn't be cut anymore.
Aristotle: Challenged Democritus' ideas.
Suggested that matter was made of four elements: earth, water, air, and fire.
1.2 The Role of Alchemists
Contributions to Chemistry:
Introduced laboratory equipment and the practice of careful observation.
Experimented with substances and transformations (e.g., the quest for the Philosopher's Stone, believed to turn lead into gold).
1.3 John Dalton (Early 1800s)
Atomic Theory
All matter is composed of extremely small particles called atoms
Atoms of a given element are identical in size, mass and other properties
Incorrect: Isotopes exist, where atoms of the same element have different masses because of neutrons
Atoms of different elements differ in size, mass and other properties.
Atoms cannot be subdivided, created or destroyed in a chemical reaction.
Incorrect: Atoms are made of subatomic particles and can split in nuclear reactions.
Atoms of different elements combine in simple whole number ratios to form chemical compound.
Law of Definite Composition/Definition Proportions/Proust Law:
A compound always contains the same elements in the same fixed ratio by mass
Importance: Ensures that compounds always have the same elements in the same proportions, allowing scientists to predict their properties and accurately perform experiments.
Law of Conservation of Mass:
Matter cannot be created or destroyed in a chemical reaction
The total mass of the reactants equals the total mass of the products.
1.4 J.J. Thomson (1897)
Cathode Ray Experiment
Discovered electrons by applying electric and magnetic fields to a cathode ray tube.
Identified electrons as negatively charged particles.
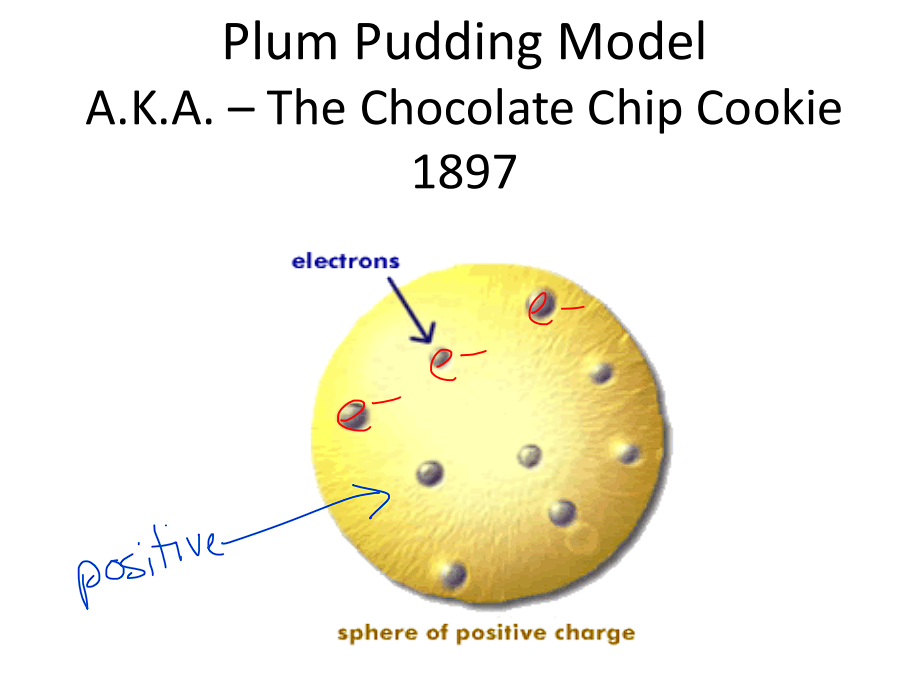
Plum Pudding Model
Proposed that atoms consist of a positively charged matrix with negatively charged electrons embedded within, like plums in a pudding.
Diagram:
1.5 Ernest Rutherford (1909)
Gold Foil Experiment
Directed alpha particles at a thin gold foil.
Most particles passed through, but a few were deflected at sharp angles.
Conclusion: Atoms are mostly empty space with a dense, positively charged nucleus.
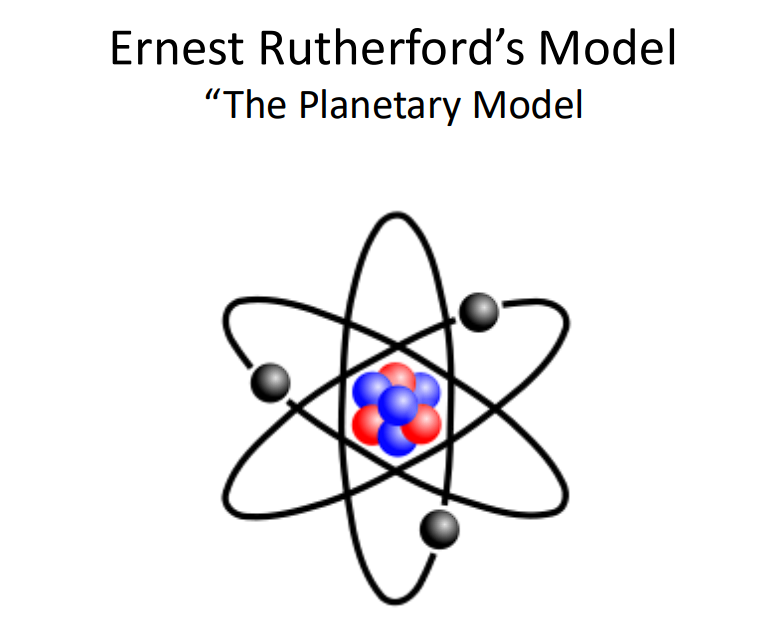
Planetary Model
Electrons orbit the nucleus in paths, similar to planets orbiting the sun.
Diagram:
1.6 James Chadwick (1932)
Discovery of the Neutron:
Chadwick discovered the neutron, an uncharged particle located in the nucleus of the atom.
He achieved this by bombarding beryllium with alpha particles, producing a new, neutral radiation that he identified as neutrons.
Impact on Atomic Theory:
Neutrons explained the existence of isotopes, as they contribute to an atom's mass without affecting its charge.
This discovery completed the modern understanding of the atom's subatomic structure (protons, neutrons, electrons).
1.7 Niels Bohr (1913)
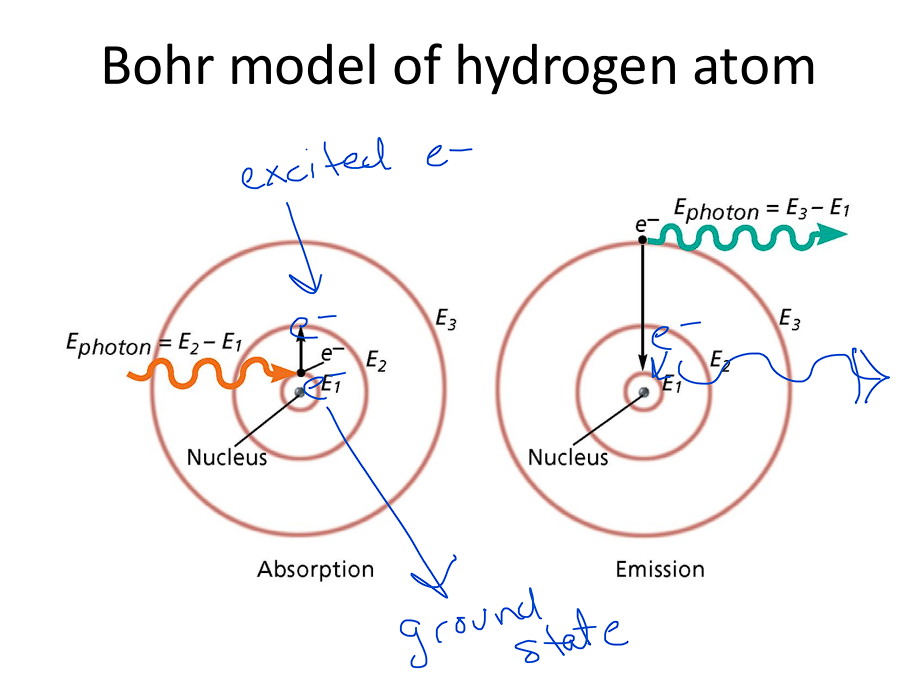
Bohr Model
Bohr Model Stated:
Hydrogen atoms exist in only specified energy states
Proposed electrons exist in quantized energy levels (specific orbits)
Hydrogen atoms can absorb only certain amounts of
energy
When excited Hydrogen atoms lose ENERGY, they lose only
certain amounts of ENERGY
This ENERGY is emitted as photons (light)
The different photons produce the different color
emission lines
The greater the jump the greater the
energy of the photons (light)
Problem with Bohr Model: Only works for hydrogen
We can apply to other atoms with math or something idk
Bohr Model:
2.1 Subatomic Particles
Protons:
Charge: +1
Mass: ~1 amu
Location: Nucleus
Neutrons:
Charge: 0 (neutral)
Mass: ~1 amu
Location: Nucleus
Electrons:
Charge: -1
Mass: ~1/1836 amu (negligible)
Location: Orbitals around the nucleus
2.2 Structure of the Atom
Nucleus: Contains protons and neutrons; accounts for most of the atom’s mass.
Electron Cloud: The region around the nucleus where electrons are likely to be found.
2.3 Quantum State and Nuclear Decay
Quantum Model of the Atom Development:
de Broglie suggests electrons can be considered as waves, introducing the dual wave-particle nature of electrons.
Schrödinger and Heisenberg contributed to this model with:
Heisenberg's Uncertainty Principle: Can't know the position and momentum of an electron at the same time.
Schrödinger further developed the idea of energy levels for atoms instead of orbits using wave mechanics to create quantum states.
Schrodinger Cat Experiment: A thought experiment where a cat is both alive and dead until observed, showing quantum superposition
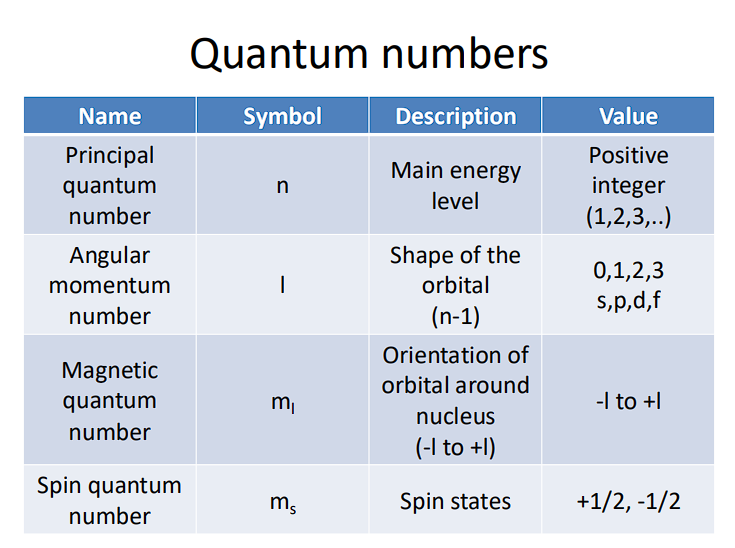
Four Quantum States/Numbers:
3 Types Of Nuclear Decay:
Alpha Decay:
The radioactive decay of an atomic nucleus by emission of an alpha particle (two protons bound to two neutrons). When an element undergoes alpha decay, its atomic number decreases by two.

Beta Decay:
The radioactive transformation of an atomic nucleus accompanying the emission of an electron. It involves unit change of atomic number but none in mass number.

Positron Emission (beta plus decay):
A proton turns into a neutron, emitting a positron (positive electron). The atomic number decreases by 1, but the mass stays the same.
Gamma Radiation:
A stream of high-energy electromagnetic radiation given off by an atomic nucleus undergoing radioactive decay.
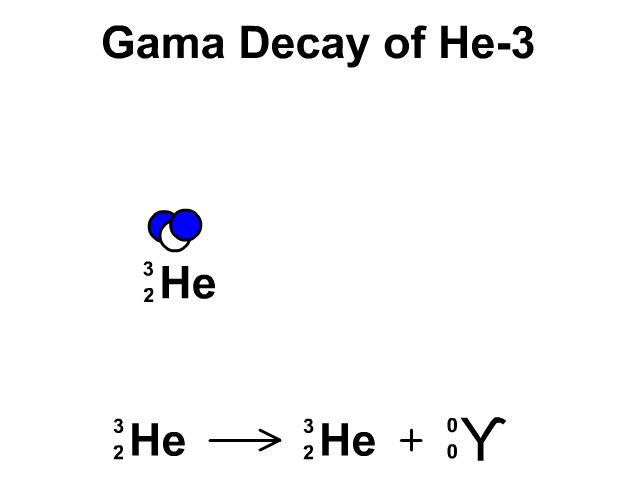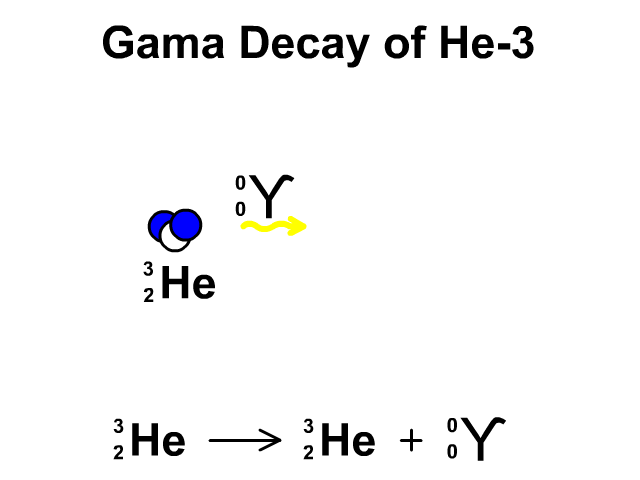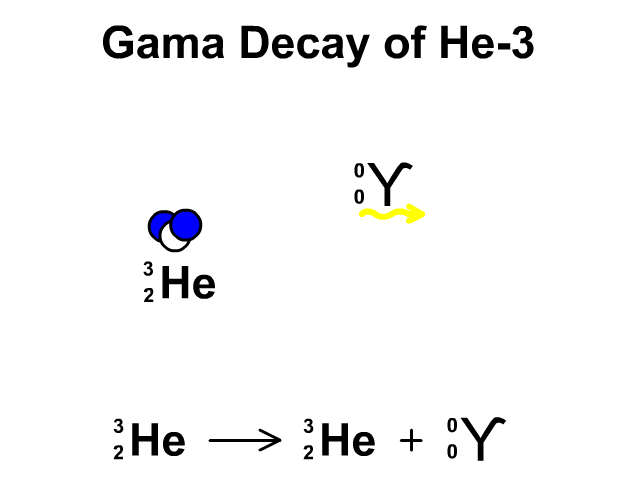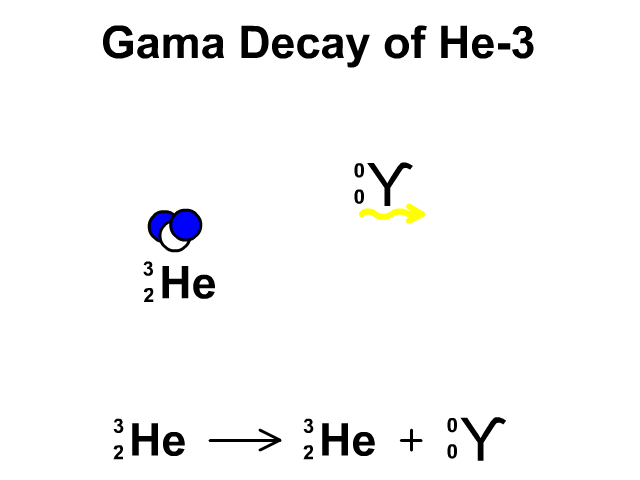
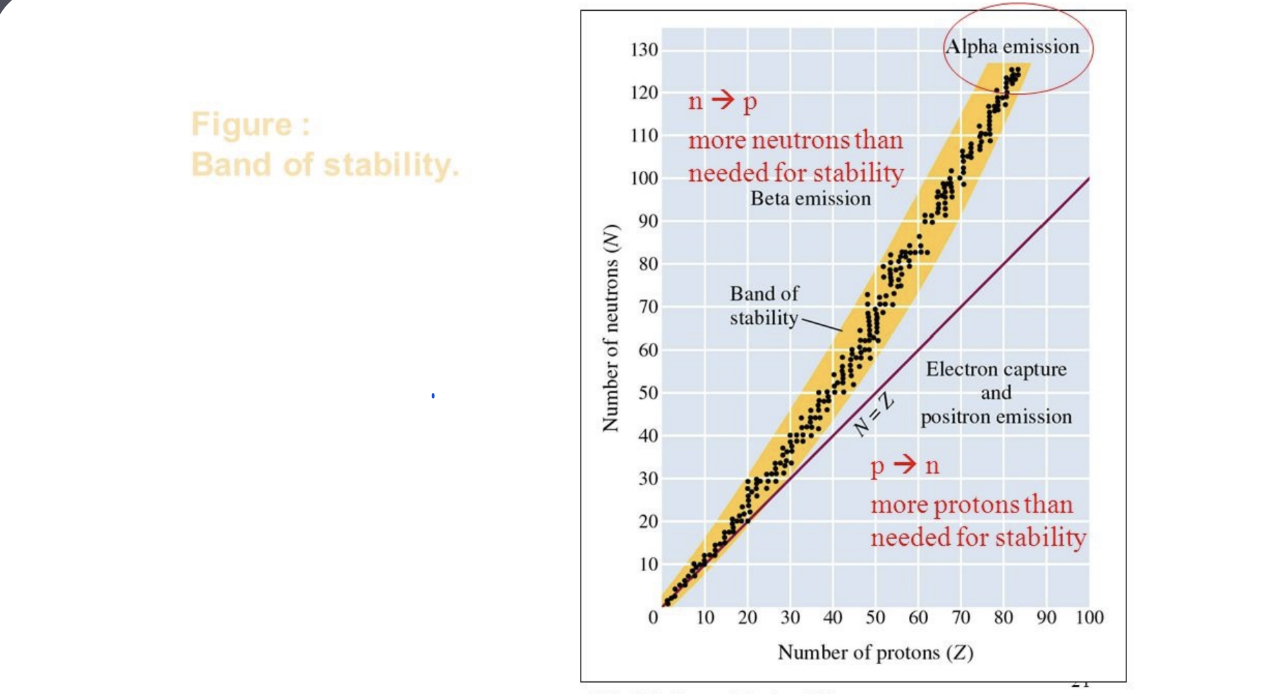
2.4 Nuclear Stability

3.1 Definition of Isotopes
Variants of an element that have the same number of protons but different numbers of neutrons.
Example:
Carbon-12: 6 protons, 6 neutrons.
Carbon-14: 6 protons, 8 neutrons.
3.2 Determining Atomic Characteristics
Atomic Number (Z): Equals the number of protons. (e.g., Carbon: Z = 6)
Atomic Mass (A): Total number of protons and neutrons.
3.3 Calculating Neutrons and Electrons
Number of Neutrons: A - Z.
Example: Carbon-12 → 12 - 6 = 6 neutrons.
Number of Electrons in Neutral Atoms: Equal to the number of protons.
Charge:
Neutral Atom: Equal protons and electrons.
Positive Ion: More protons than electrons.
Negative Ion: More electrons than protons.
4.1 Formula
Multiply the mass of each isotope by its relative abundance, then sum the values.
Example Calculation:
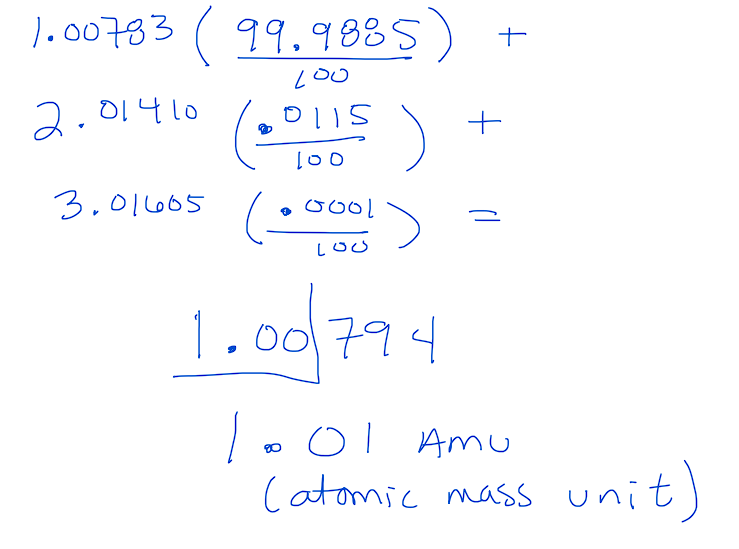
4.2 Percent Abundance
Formula:

5.1 Key Definitions
Wavelength (λ): Distance between successive crests of a wave, measured in meters (m). Peak/crest is the highest point and trough is the lowest point
Frequency (ν): Number of wave cycles passing a point per second, measured in Hertz (Hz).
Energy (E): The capacity to do work, which can be calculated with relevant formulas.
Properties | Definition | Unit |
Wavelength | Distance between consecutive crests or troughs | meters (m) |
Frequency | Cycles per second | Hertz (Hz) or S-1 |
Energy | Capacity to do work | Joules (J) |
5.2 Formulas
Relationship between Energy, Frequency, and Wavelength:
E = energy (Joules, J)
h = Planck's constant (6.626×10-34 Js)
ν = frequency (Hz)
Energy Formula:
E = hν
Where h (Planck's constant) = 6.626 x 10⁻³⁴ Js.
Wave Equation:
c = λν
Where c (speed of light) = 3.00 x 108 m/s.
5.3 Relationships
Inversely Proportional:
As wavelength (λ) increases, frequency (ν) decreases
Longer wavelengths correspond to lower energy and low frequency
Directly Proportional:
As frequency (ν) increases, energy (E) increases.
Higher frequency leads to higher energy
6.1 Methods of Writing Configurations
Orbital Notation:
A visual representation using arrows to depict electrons in specific orbitals (e.g., ↑↓ for paired electrons in 1s)
Example:
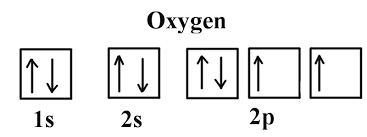
Full Electron Configurations:
Detailed listing of electrons in their respective subshells.
Example for Oxygen (O): 1s² 2s² 2p⁴
Noble Gas Configuration:
Simplifies full configurations by substituting the last noble gas.
Example for Sodium (Na): [Ne] 3s1
6.2 Quantum Numbers

6.3 Order of Filling Orbitals
Aufbau Principle:
Electrons occupy orbitals starting with the lowest energy level before filling higher levels.
Example: Example: The 1s orbital is filled before 2s, which is filled before 2p.
Why this occurs: Orbitals closer to the nucleus (lower principal quantum numbers) are more stable due to stronger electrostatic attraction between the electrons and the nucleus.
Pauli Exclusion Principle:
No two electrons in the same atom can have the same set of four quantum numbers (n, l, ml, ms)
Each orbital can hold a maximum of two electrons with opposite spins (↑↓).
Why this occurs: Opposite spins minimize electron-electron repulsion within the same orbital, stabilizing the atom.
Hund’s Rule:
Electrons fill orbitals of the same energy (degenerate orbitals) singly, with parallel spins, before pairing up.
Example for Nitrogen (N):
1s2 2s2 2p3
In the 2p subshell, the three electrons will occupy three separate orbitals (↑, ↑, ↑) before any pairing occurs.
Why this occurs: Spreading out electrons minimizes electron-electron repulsion, which makes the atom more stable.
6.4 Special Exceptions
D-block Exceptions:
For d⁴ and d⁹ configurations, one electron is moved from the s orbital to the d orbital to make the d-subshell more stable.
Why: Half-filled (d5) and fully filled (d10) d-subshells are more stable due to reduced electron repulsion and symmetry.
What to do:
If a configuration ends in d4: Change it to d5 by taking one electron from the s orbital.
Example: Chromium (Cr): [Ar] 4s1 3d5 (not [Ar] 4s2 3d4)
If a configuration ends in d9: Change it to d10 by taking one electron from the s orbital.
Example: Copper (Cu): [Ar] 4s1 3d10 (not [Ar] 4s2 3d9)
F-block Configurations:
The f-block (lanthanides and actinides) involves filling 4f or 5f orbitals.
What to do:
Fill the s orbital first, then the f orbitals, followed by the d orbitals if needed.mn. .
Example: Cerium (Ce): [Xe] 6s2 4f1 5d1
Key Tip: Always follow the order of filling based on the Aufbau principle, even if it seems complicated.
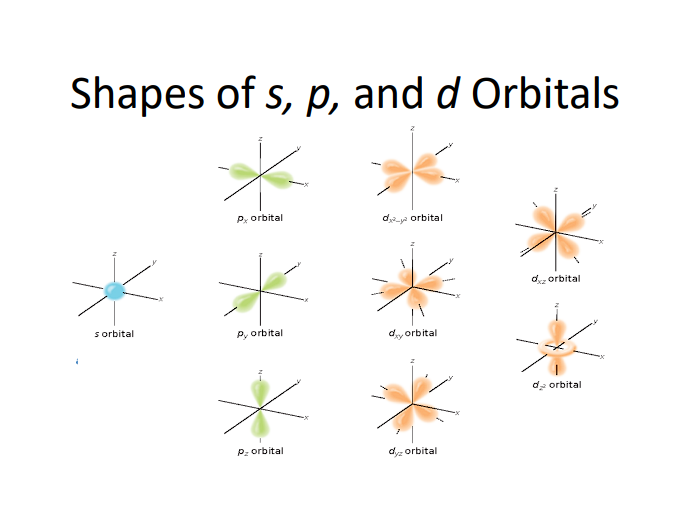
6.5 Allowed Orbitals & Shapes
Shapes of Orbitals:
s-orbital: Spherical, holds up to 2 electrons.
p-orbital: Dumbbell-shaped, holds up to 6 electrons (three p-orbitals).
d-orbital: Cloverleaf shape, holds up to 10 electrons (five d-orbitals).
f-orbital: Complex shapes, holds up to 14 electrons (seven f-orbitals).
Shapes of s, p, and d Orbitals
F Orbitals
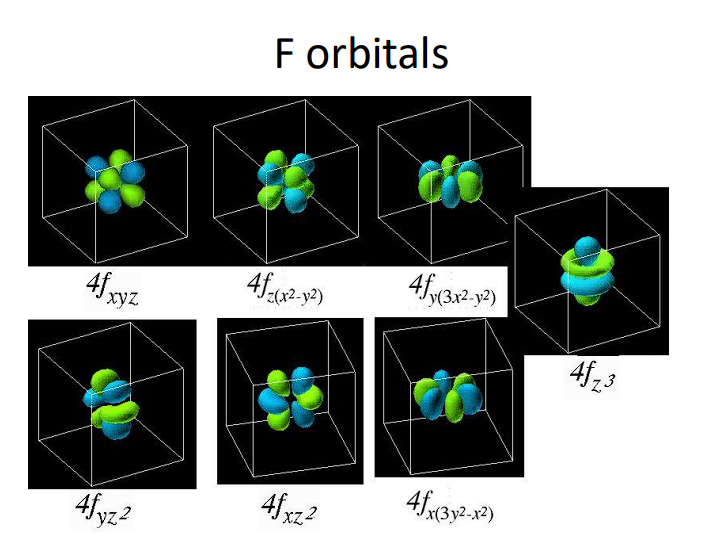
6.6 Valence Electrons and Isoelectronic Species
Valence Electrons:
Outermost electrons that play a key role in chemical bonding;
Isoelectronic Species:
Atoms or ions with identical electron configurations (e.g., Na⁺ and Ne).
How to find Valence Electrons:
Determine the Electron Configuration: This shows how the electrons are distributed among the orbitals.
For example, the electron configuration for Oxygen (O) is 1s² 2s² 2p⁴.
Identify the Outermost Shell: The electrons in the outermost shell (highest principal energy level) are the valence electrons. In the example of Oxygen, the outermost shell is the second shell (n=2).
Count the Valence Electrons: Add the electrons in the outermost shell orbitals. For Oxygen, the 2s² and 2p⁴ mean there are 6 valence electrons (2 from 2s and 4 from 2p).
READ THE INSTRUCTIONS AT THE BEGINNING OF THE TEST FOR SIG FIGS