Atomic Physics
Atoms consist of:
- Nucleus: central part of atom made of protons (positively charged) and neutrons. These two types of particles are called nucleons. They are bound together by the strong nuclear force.
- Electrons: almost mass-less particles which orbit nucleus in shells
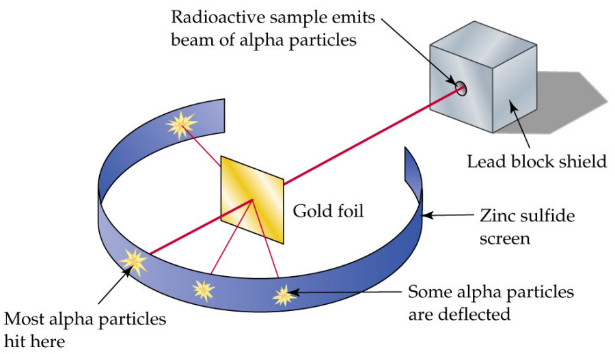
This is proved by Rutherford’s Gold Foil Experiment2
Proton number: number of protons in an atom
Nucleon number: the number of nucleons (protons + neutrons) in an atom
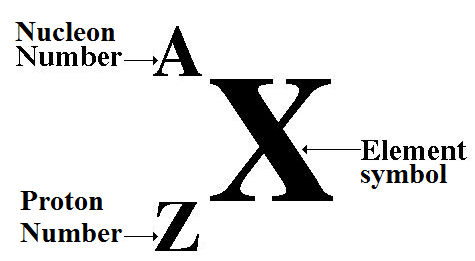
Isotope: Atoms of the same element that have different numbers of neutrons e.g. Carbon 12 and Carbon 14.
- There are non-radioactive isotopes and radio-isotopes.
- Radio isotopes are unstable atoms, which break down giving radiation
Uses:
- Medical use: cancer treatment (radiotherapy) – rays kill cancer cells using cobalt-60
- Industrial use: to check for leaks – radioisotopes (tracers) added to oil/gas. At leaks radiation is detected using a Geiger counter.
Detection of Radioactivity
Background radiation: small amount of radiation around us all time because of radioactive materials in the environment.
It mainly comes from natural sources such as soil, rocks, air, building materials, food and drink
A Geiger-Müller (GM) tube can be used to detects alpha, beta and gamma radiation
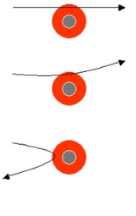
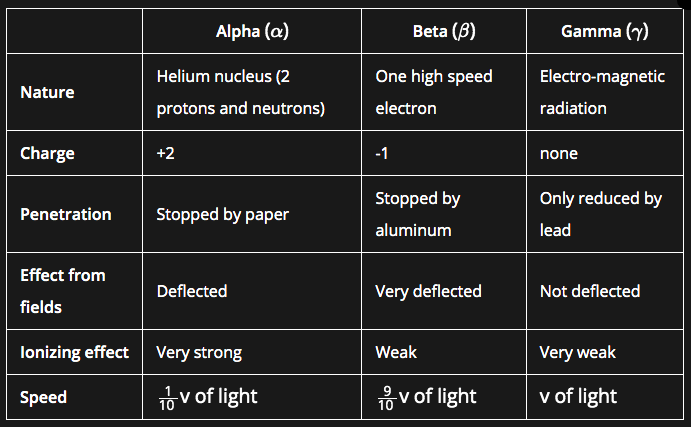
- Type of Radioactive Emissions:
Radioactive Decay
- Radioactive decay: A radioisotope (unstable arrangement of neutrons and protons) is altered to make a more stable arrangement.
- The parent nucleus becomes a daughter nucleus and a particle (decay products).
- The nucleus changes when undergoing alpha or beta decay
Alpha decay:
- An element with a proton number 2 lower and nucleon number 4 lower, and an alpha particle is made (2p + 2n)
e.g. Radium-226 nucleus → Radon-222 + helium-4 nucleus
Beta decay:
- A neutron changes into a proton, an electron and an antineutrino so an element with the same nucleon number but with a proton number 1 higher e.g.
e.g. iodine-131 → xenon-131 + antineutrino + beta particle
Gamma emission:
- Gamma emission by itself causes no change in mass number or atomic number; they just emit energy
- Some isotopes do not change in mass or atomic number however they emit energy as their particles rearrange themselves to become more stable
Half Life
- Half-life of a radioisotope: is the time taken for half the nuclei present in any given sample to decay.
- Remember to factor background radiation in half-life calculations involving tables and decay curves!
Safety Precautions
- Radioactive material is stored in a lead container
- Picked up with tongs, not bare hands
- Kept away from the body and not pointed at people
- Left out of its container for as short a time as possible
Rutherford’s Experiment
Thin gold foil is bombarded with alpha particles, which are positively charged.
Most passed straight through, but few were repelled so strongly that they were bounced back or deflected at large angles.
Rutherford concluded that the atom must be largely empty space, with its positive charge and most of its mass concentrated in a tiny nucleus.