AP Bio Unit 1
Important elements to life (CHNOPS):
Carbon, Hydrogen, Nitrogen, Oxygen, Phosphorus, Sulfur
Living organisms are made up of chemicals based mostly on the element carbon
Carbon is unparalleled in its ability to form large, complex, varied molecules
A compound containing carbon is an organic compound
Trace elements- very small amounts but essential to function
Iron (Fe), sodium (Na+), potassium (K+), copper (Cu), Iodine (I)
Important molecules of all living things fall into 4 main classes:
Carbohydrates (energy)- Carbon
Lipids (long-term energy)- Carbon, Phosphorus
Proteins (muscles)- Carbon, Sulfur, Nitrogen
Nucleic acids (store/transmit genetic information)- Carbon, Nitrogen, Phosphorus
Carbon
Why carbon?
Abundant, versatile in bonds, tetravalent- makes 4 bonds to get stable = infinite variety
Valence electrons—electrons on outermost energy level
Valence—number of covalent bonds an element can make
Carbon-4, Hydrogen- 1, Oxygen- 2, Nitrogen- 3
*STRUCTURE affects molecule function
Ex) Ismoers- molecules with the SAME molcular formula but different structures/order—emergent property a property that only occurs at a specific level
Three kinds of isomers:
Structural- same chemical formula different order/arrangement
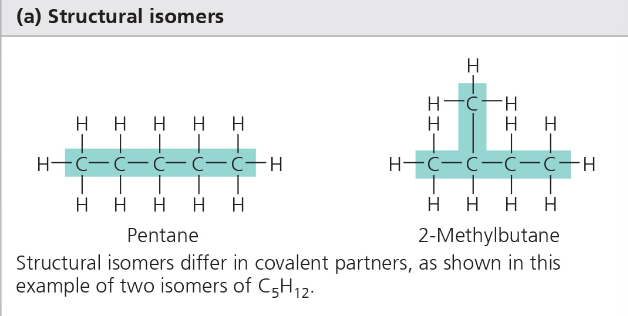
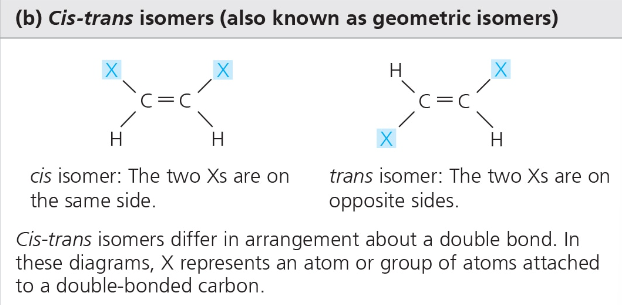
Cis-Trans- same formula, different positioning around a double bond
Cis- Xs arranged on the same side of a double bond
Trans- Xs arranged on opposite sides of the double bond
Enantiomers- same formula, mirror image positioning around a central carbon due to an asymmetric carbon attached to 4 different atoms
Left-handed and Right-handed variations
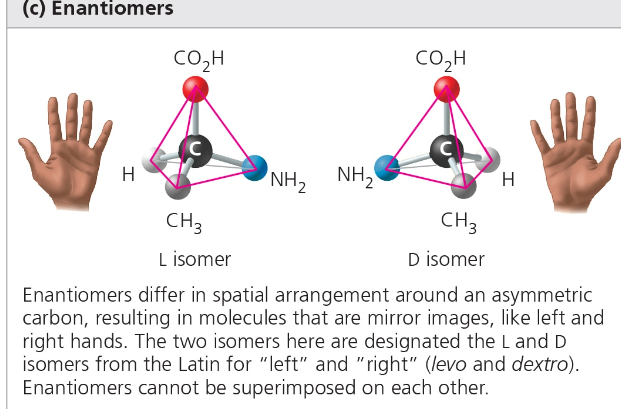
Biological processes tend to use one or two of the enantiomer forms
Ex. R-Ibuprofen vs S-Ibuprofen (works to reduce pain)
Hydrocarbons- organic molecules consisting of only carbon and hydrogen
*ORGANIC substances have hydrocarbons
Many organic molecules such as fats have hydrocarbon components
Hydrocarbons can undergo reactions that release a large amount of energy
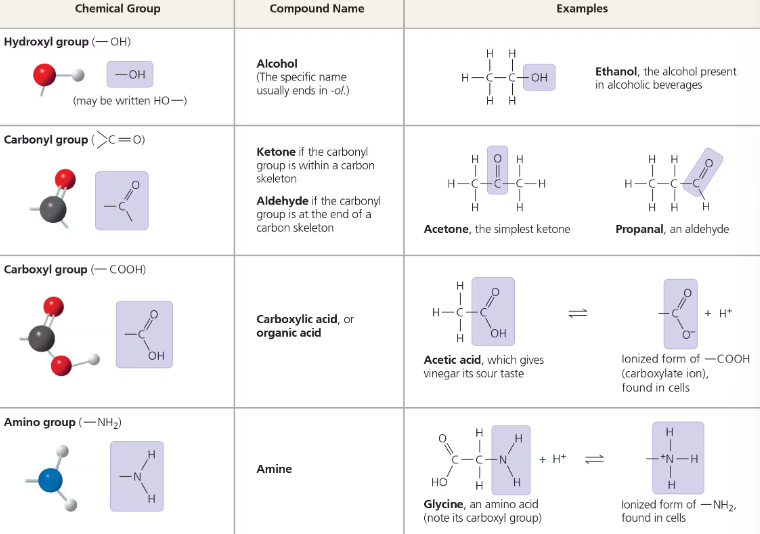
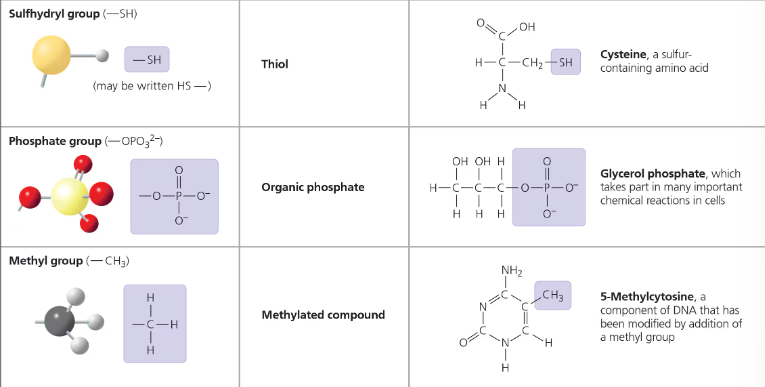
Chemical groups most important to life:
Carbs: Hydroxyl group, Carbonyl group
Amino Acids/Proteins: Carboxyl group, amino group, hydroxyl group
Nucleic Acid: Phosphate group, Hydroxyl group (sugar), Carbonyl group (nitrogen base)
Lipids: Hydroxyl group (glycerol), Carboxyl group (fatty acid chains), Methyl group (fatty acid chains)
Each functional group participates in chemical reactions in a characteristic way
1.2 Elements of Life
Living systems require a constant input of energy
Law of conservation of energy: energy cannot be created nor destroyed only TRANSFORMED
Living systems follow the laws of energy
Living systems need constant input of energy to grow, reproduce, maintain organization
Living systems mainly use energy stored in chemical bonds
Living systems require an exchange of matter
Atoms/molecules from the environment needed to build new molecules
Carbon is used to build all 4 biological molecules (carbs, proteins, nucleic acids, lipids)
Nitrogen used to build proteins and nucleic acids
Phosphorus used to build nucleic acids and some lipids
Carbon is used to build macromolecules
Carbon’s unique ability to bond w/ other carbon atoms creating carbon skeletons other atoms can attach to
Enables creation of large and complex molecules
Carbon contains molecules that can be used to store energy
Carbon containing molecules can be used to form basic cell structures
1.3 Intro to Biological Macromolecules
Monomers: Chemical subunits used to create polymers
Monomers have specific chemical properties allowing them to interact with one another
Covalent bond is formed between two interacting monomers
Polymers: macromolecule (large moelcule) made of many monomers
Polymers are specific to the monomers they consist of
Ex. Monosaccharide → Carbohydrate (Polysaccharide) ; Amino acid → Protein; Nucleotide → Nucleic Acid; Fatty Acid → Lipids (lipids don’t have true monomers)
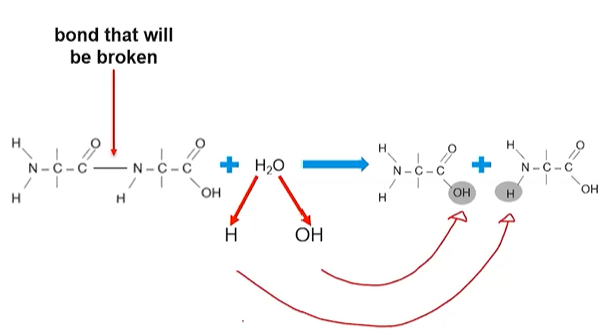
Dehydration Synthesis reactions (Condensation reactions) form covalent bonds
Dehydration synthesis reactions create macromolecules
Subcomponents of a water molecule (H and OH) are removed from interacting monomers and a covalent bond forms
The H and OH join to form a molecule of water, water is a byproduct of this reaction
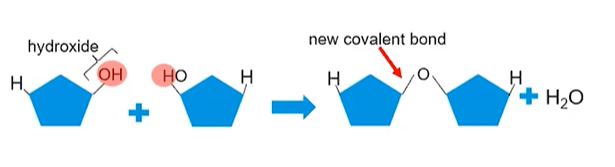
Ex. Dehydration Synthesis creates carbonhydrates
Carbohydrate monomers have hydroxides (OH) and hydrogen atoms (H) attached
One monomer loses an entire hydroxide while the other will only lose the hydrogen to form hydroxide
A covalent bond will form where the hydroxide/hydrogen atom were REMOVED
Hydroxide and hydrogen join forming a water molecule
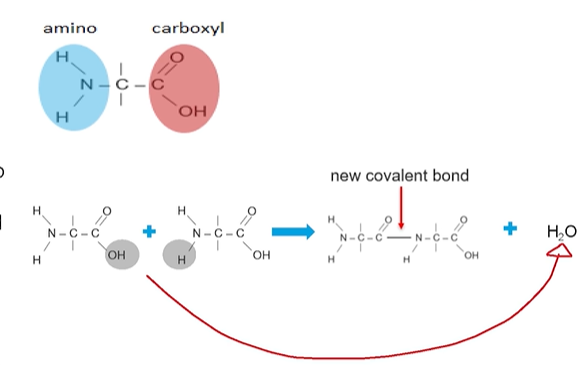
Dehydration synthesis creates proteins
Each amino acid has an amino group (NH2) terminus and a carboxyl group (COOH) terminus
A hydroxide is lost from the carboxyl group and hydrogen atom is lost from the amino group of another amino acid
A covalent bond/peptide bond forms between the monomers where the hydrogen/hydroxide were removed
The hydroxide and hydrogen atoms form a water molecule
Hydrolysis reactions cleave covalent bonds
Polymers are hydrolyzed (broken down) into monomers during a hydrolysis reaction
Covalent bonds between the monomers are cleaved (broken) during a hydrolysis reaction
A water molecule is hydrolyzed into subcomponents (H and OH) and each added to a different monomer
Ex. Proteins undergo hydrolysis reactions
Covalent bonds between amino acids can be cleaved (broken)
A water molecule is hydrolyzed and each subcomponent of water (H and OH) will be bonded to different amino acids
Result in separate amino acid monomers
1.4 Properties of Biological Molecules
Living organisms are organized in a hierarchy of structural levels
At every level of organization function is related to structure
A change in the structure reesults in a change in the function
Properties determined by structure and function of molecules
Nucleic Acids
**DO NOT CONFUSE W/ AMINO ACIDS
Nucleic acids—polymers comprised of monomers called nucleotides
Basic structure containing 3 subcomponents: 5-carbon (pentose) sugar, a phosphate group, a nitrogen base
Store biological information in the sequence of nucleotides
Ex. DNA vs. RNA
Deoxyribose (sugar) 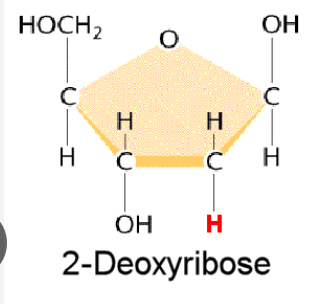 | Ribose (sugar) 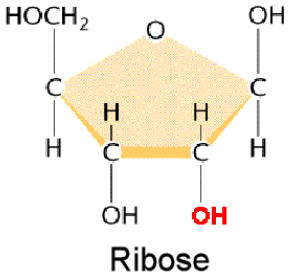 |
Nitrogen bases: Thymine, Adenine, Guanine, Cytosine | Nitrogen bases: Uracil, Adenine, Guanine, Cytosine |
Amino acids- monomers that make up proteins
Have directionality with an animo (NH2) group and carboxyl (COOH) group
Polypeptide- primary structure; consists of a specific order of amino acids → determines the overall shape and function of the protein
R-group- group of atoms attached to the central carbon differs amino acids from one another
R-groups can be Hydrophobic, Hydrophilic, or Ionic
Protein can have different amino acids in the polypeptide allowing the protein to have regional differences in structure/function
Carbohydrates
Complex carbohydrates can have monomers whose structures determine the properties and functions of the carbohydrate
Lipids
Nonpolar macromolecules DO NO HAVE TRUE MONOMERS comprised of subunits (fatty acids and glycerol)
Fatty acid components determine structure/function based on SATURATION
Saturated: no double bond; Unsaturated: double bond between a carbon group
Specialized phospholipids- contain BOTH hydrophilic (polar head)+ hydrophobic (nonpolar tail) regions determine interactions with other molecules
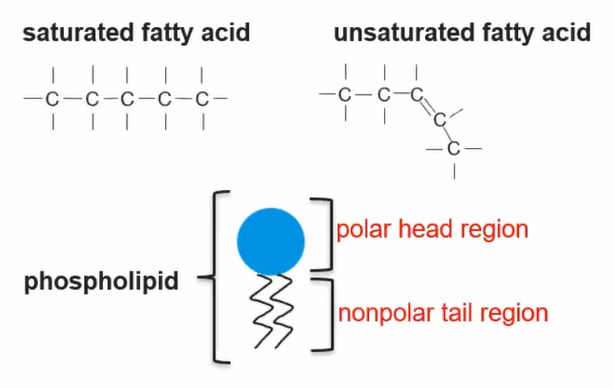
Cell membranes contain lipids + proteins
Phospholipids and some membrane proteins have both hydrophilic/hydrophobic regions
Hydrophilic regions can interact with each other and the water environments (facing outwards)
Hydrophobic regions can interact with each other but NOT water environments (facing inwards)
1.5 Structure and Function of Biological Macromolecules
Directionality in subunits influences structure of nucleic acid polymers
Linear sequence of all nucleic acids characterized by a 3’ hydroxyl and 5’ phosphate of the sugar in the nucleotide
Ex. DNA is nucelic acid plymer containing TWO strands each in an antiparallel 5’-3’ direction
Adenine - Thymine base pairs have TWO hydrogen bonds; Guanine - Cytosine held together by THREE hydrogen bonds
More hydrogen bonds = more stable the molecule’s structure is
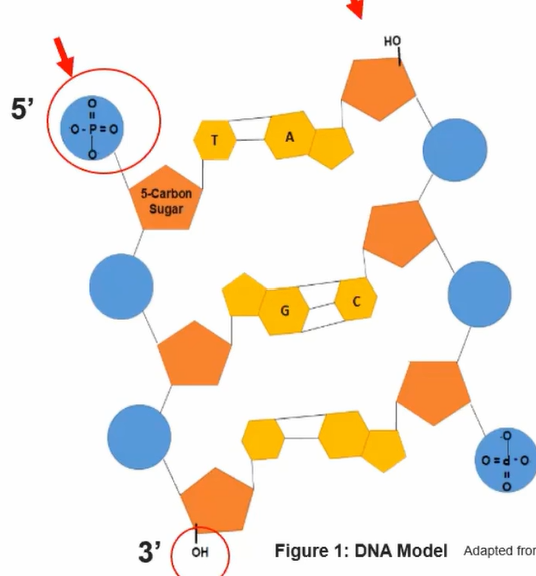
Linear sequence of nucleotides encodes biological information
Any change in sequence may change encoded information
Synthesis:
Nucleotides can only be added to the 3’ end
Covalent bonds used to connect free nucelotides to the strand
Antiparallel Structure effect of replication: Since nucleotides can only be added to the 3' end, new nucleotides are added to the DNA strand moving from the 5' to 3' direction (leading strand)
Meanwhile, on the opposite strand, since nucleotides are still added from the 5' to 3' direction yet the strand runs opposite starting from the 3' to 5' direction, it replicates starting from the opposite direction (lagging strand)
Thus, short segments called Okazaki fragments are created that are later joined together.
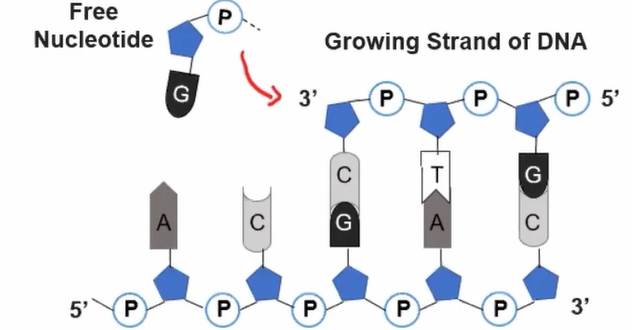
Direcitonality and protein structure:
Proteins comprise linear chains of amino acids that have a directionality with the amino + carboxyl groups
New amino acids added to carboxyl group connected by covalent bonds at the carboxyl group of the growing peptide chain
Elements of protein structure
Primary structure- determined by sequence of amino acids held by covalent (peptide) bonds
Secondary structure- local folding of amino acid chain into alpha-helices/beta-sheets
Tertiary structure- overall 3D shape of the protein and often minimizes free energy; various types of bonds between R-groups stabilize protein
Quaternary structure- arises from interactions between multiple polypeptide units
Directionality and structure of carbohydrates
Carbodydrates comprise linear chains of sugar monomers connected by covalent bonds
Small directional changes in compnents (i.e. direction of OH group) can result in functional differences
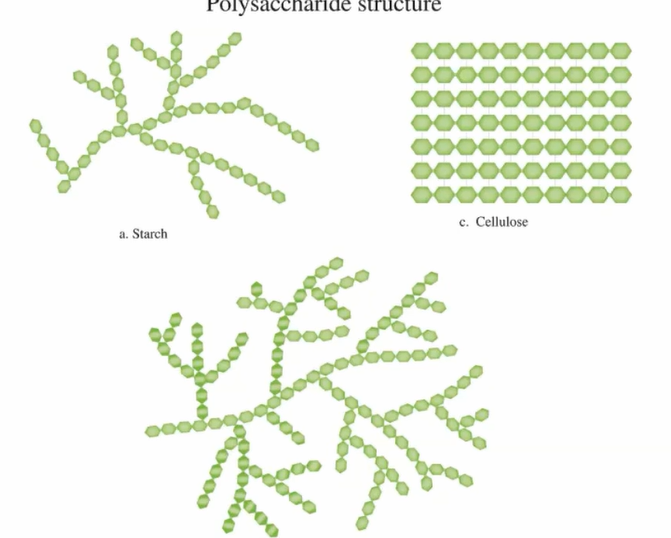
Carbohydrate polymers can be linear or branched
Starch and glycogenboth function in energy storage (starch-plants; glycogen-humans/vertebrates)
Cellulose provides support and strength to cell walls
Carbon (Textbook ch. 3.1)
Carbon has 4/8 valence electrons in its outer shell and a valence of 4 → enables carbon to form large, complex molecules
Valence: the number of covalent bonds an atom can form
Carbon-4, Oxygen-2, Nitrogen-3, Hydrogen-1
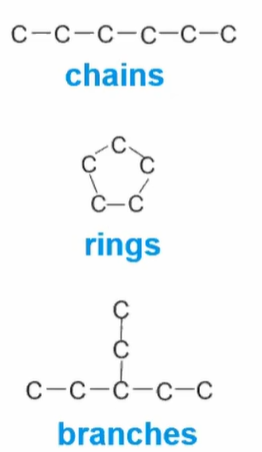
Carbon can bond to various atoms including other carbon atoms to form carbon skeletons of organic carbon
Shapes of carbon bonds:
Carbohydrates (Textbook Ch. 3.3)
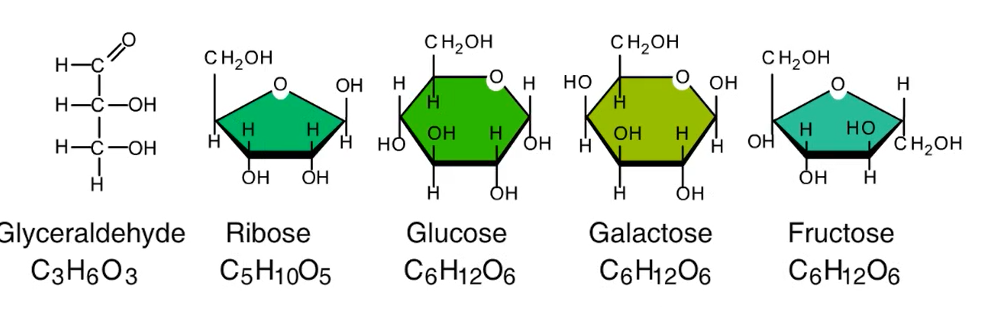
Monomer- Monosaccharides
Molecules Involved: Carbon, Hydrogen, Oxygen
Characteristics of carbohydrates/sugars:
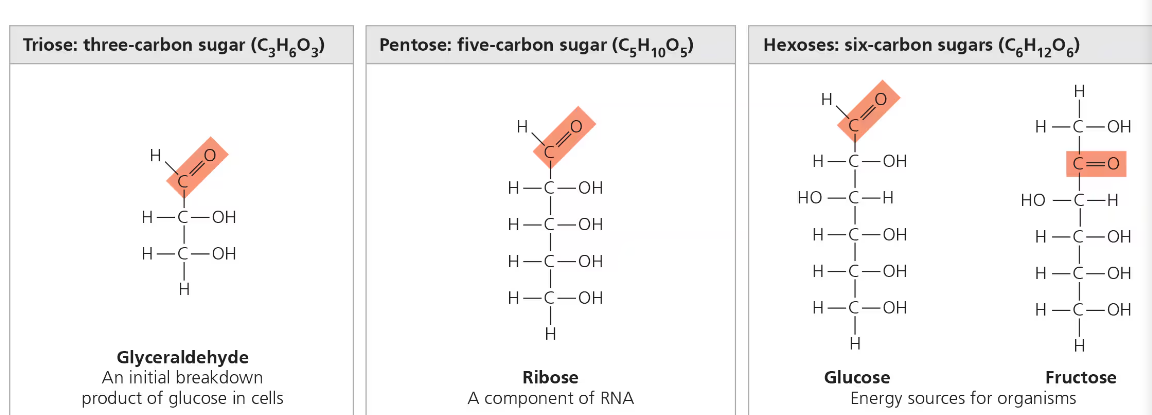
Carbon skeleton (C-C-C-C); ranges from 3-7 carbons long
Carbonyl group (C=O)
Multiple hydroxyl groups (OH)
6 carbons—Hexoses; 5 carbons—Hexoses; 3 carbons—Trioses;
Major nutrients for cells
Cells extract energy from glucose molecules by breaking them down
Carbon skeletons of monosaccharides raw material for synthesis of other types of small organic molecules (amino acids)
Examples: Glucose (C6H12O6), Galactose, Fructose, Ribose, Glyceraldehyde
Macromolecules-
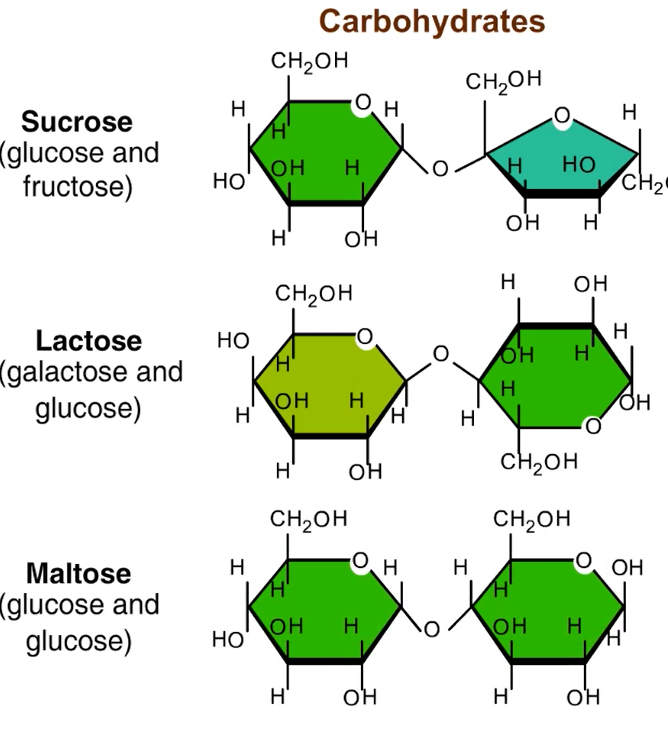
Dissacharides- 2 monosaccahrides joined by glycosidic linkage (covalent bond formed through a dehydration reaction)
Must be broken into monosaccharides to be used for energy
Examples: Sucrose (glucose + fructose); Lactose (galactose + glucose); Maltose (glucose+glucose)
Polysaccharides- many sugar building blocks joined by glycosidic linkages
Structure/function determined by sugar monomers and position of glycosidic linkages
Storage Polysaccharides: serve as storage material—hydrolyzed to provide sugar monomers for cells
Examples:
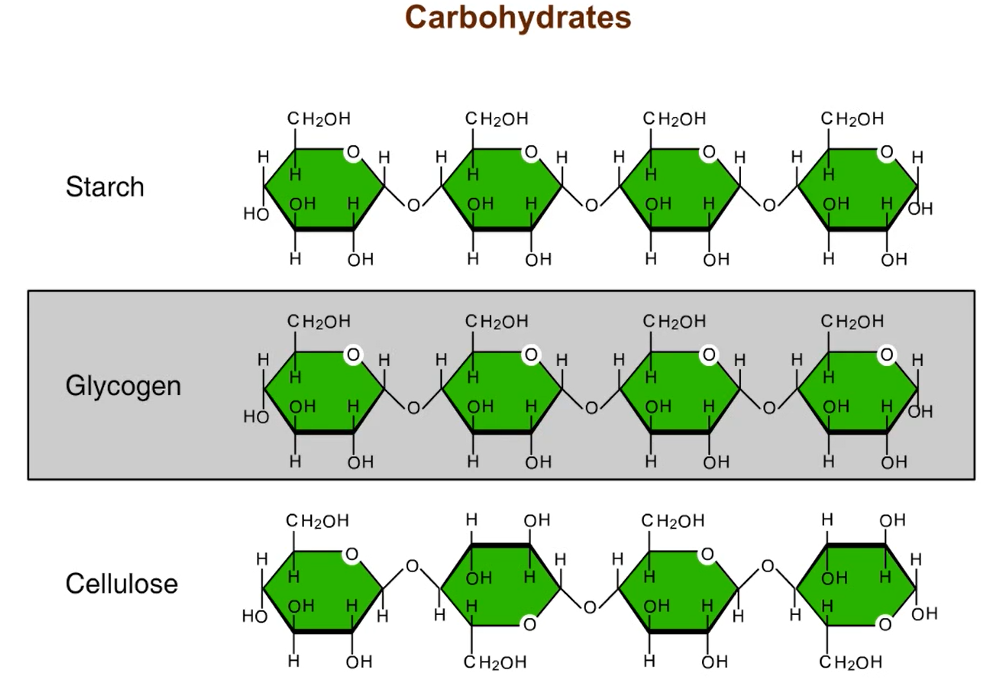
Starch stores energy—withdrawn by hydrolysis reaction breaking bonds between glucose monomers
Glycocen—stored in animal liver/muscle cells breakdown of glycogen releases glucose
Structural Polysacchrides
Serve as building material for structures to protect the cell/organism
Examples:
Chitin- used by anthropods to build exoskeletons/fungi cell walls
Cellulose- forms plant cell walls
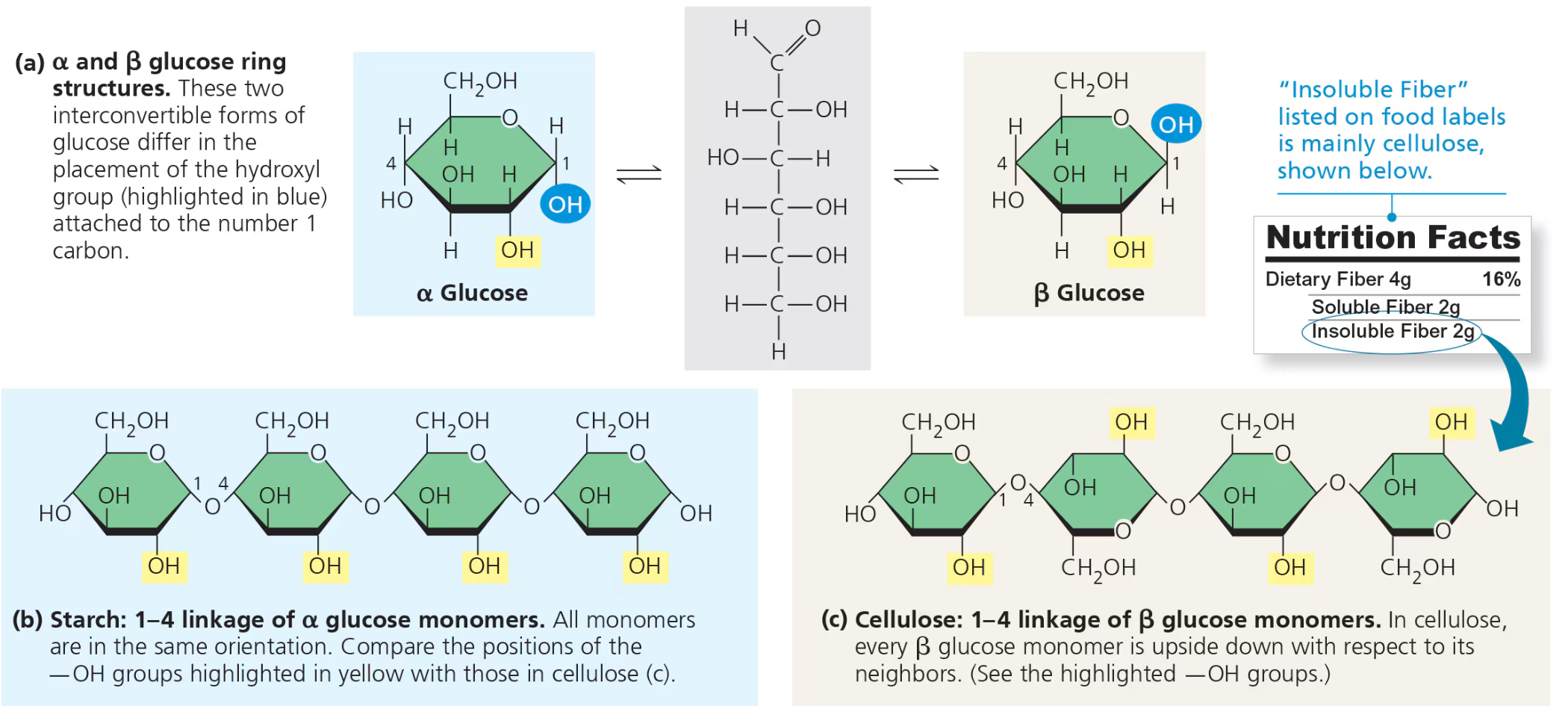
Starch vs. Cellulose
Starch and cellulose similar in structure except all glucose monomers in starch are in the alpha (α) configeration while cellulose is all in the beta (β) —making every other one appear “upside down”
Enzymes that digest starge by hydolysizing (α) linkages unable to for cellulose →
Few organisms can digest cellulose unless microorganisms in gut of animals like cows can hydrolyze cellulose
Lipids (Textbook Ch. 3.4)
Characteristics of Lipids:
NO true monomers or polymers; not big enough to be considered macromolecules
Shared characteristic: HYDROPHOBIC molecules—low solubility in water
Consist of mostly hydrocarbon (CH) regions
Purposes: Stores energy (long-term), insulates body, cushions organs (cell membrane)
Molecules involved: Carbon, Hydrogen, Oxygen, Phosphate* (Nitrogen?)
Ex. Fats, Phospholipids, Steroids
Fats
Purpose: Energy storage (stores more than carbohydrates)
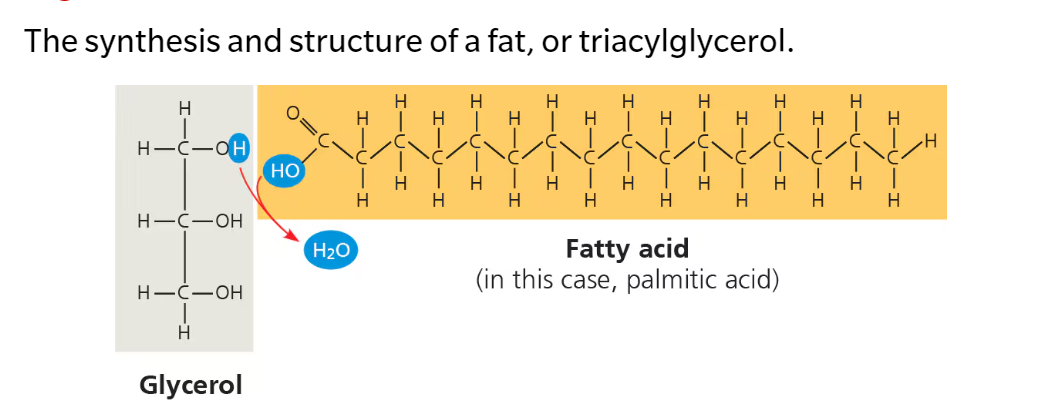
Triglycerides/Triacylglycerol: Three fatty acid tails bind to a molecule of glycerol
Glycerol- 3 carbons bearing a hydroxyl (OH) group; a type of alcohol
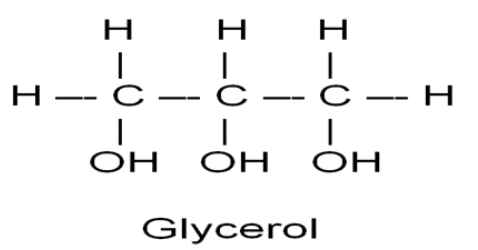
Fatty Acid- long carbon skeleton (16-18 C atoms) with one end part of a carboxyl group (COOH) and the rest consisting of a hydrocarbon chain (C-H)
Nonpolar hydrocarbon (C-H) bonds cause fatty acids to be hydrophobic
Large molecules assembled from smaller molecules through dehydration reactions
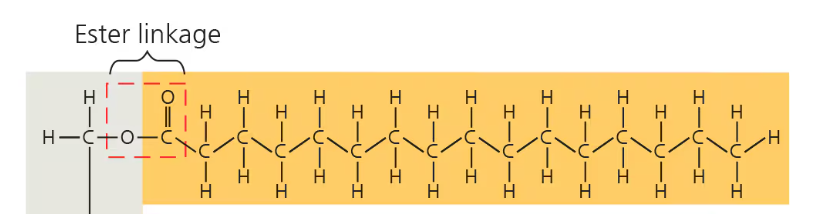
Fatty acid molecule joined to glycerol via dehydration synthesis → esther linkage—bond between a hydroxyl (OH) and carboxyl group (COOH)
Saturated Fats: Only contain single carbon bonds in hydrocarbon chains of fatty acid tails
Solids at room temperature (b/c molecules packed closer together) Ex. Butter, lard
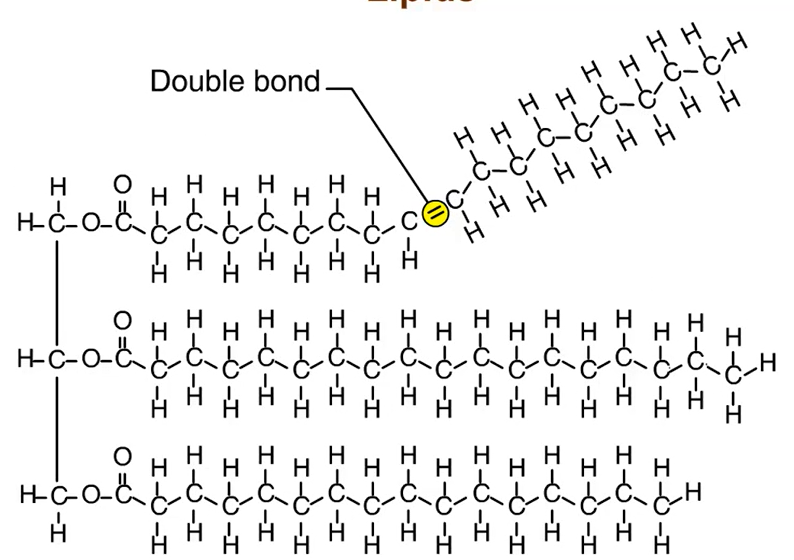
Unsaturated Fats: Contain double bonds in one or more hydrocarbon chains of fatty acids (usually cis double bonds = kink/bend in hydrocarbon chain)
Liquids at room temperature Ex. Vegetable oil
*Trans Fats: Synthetically convert unsaturated to saturated fats by adding hydrogen → produces unsaturated fats with trans double bonds
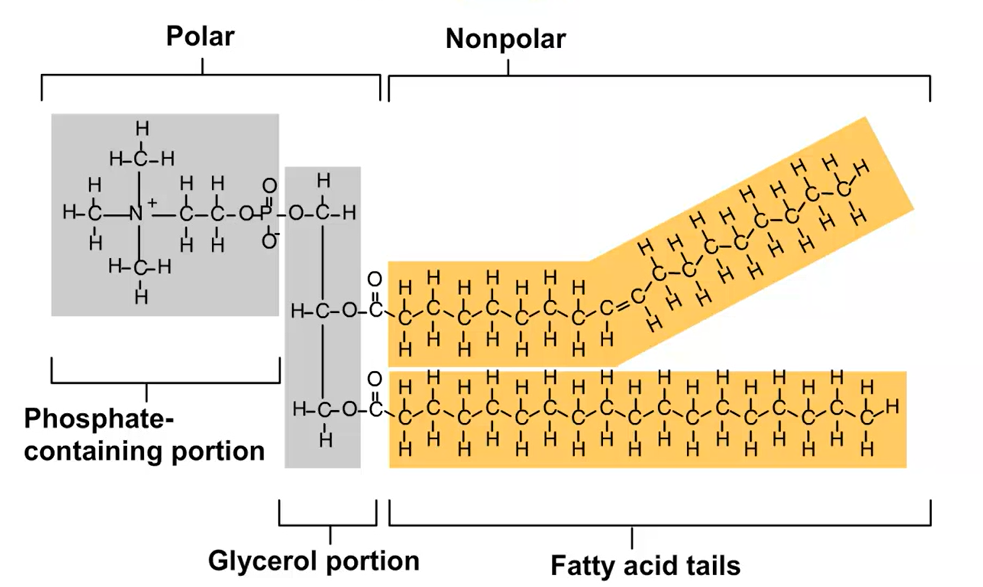
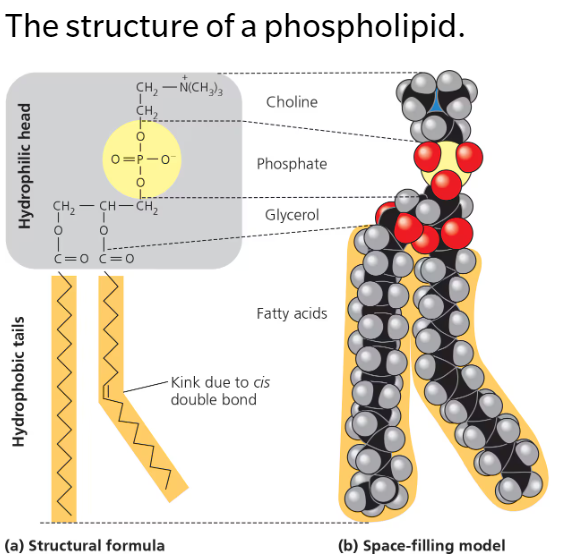
Phospholipids
Phosphate-containing polar (hydrophilic) head connected to glycerol and TWO nonpolar (hydrophobic) fatty acid tails
Head: Negatively charged phosphate group attached to glycerol may be attached to another charged molecule such as choline
Purpose: Makes up cell membranes (phospholipid bilayer)
Assemble into a double-layered sheet with polar heads facing outwards towards the water and fatty acid tails shielded from water
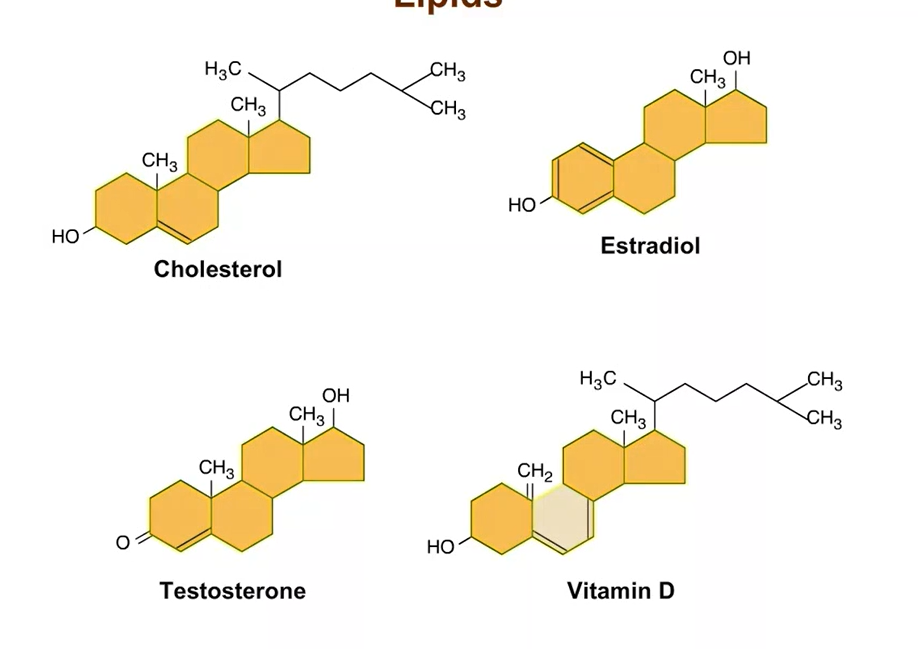
Steroids
All have carbon skeleton with 4 rings; difference in chemical groups attached to the rings
Cholesterol
Component of animal cell membranes + precursor other steroids are synthesized from
Synthesized in the liver and obtained from diet
Proteins (Textbook Ch. 3.5)
Protein- biologically functional molecule made up of one of more polypeptides folded and coiled into a 3D structure
Made of monomer amino acids linked together via peptide bonds (covalent bond) → polymer = polypeptide
Protein Functions:
Enzymatic Proteins (Enzymes)- Catalysts that speed up and chemical reactions; regulate metabolism Ex) Digestive enzymes- catalyze the hydrolysis (breakdown) of bonds in food  | Storage Proteins- Storage of amino acids Ex) Ovalbumin- protein of egg white source of amino acid for embryo 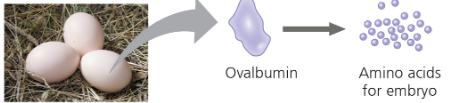 | Hormonal Proteins- coordination of organism’s activities Ex) Insulin causes other tissues to take up glucose → regulate blood sugar concentration 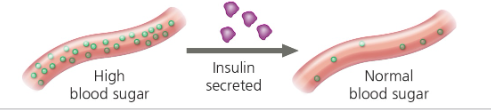 |
Contractile and motor proteins- Movement Ex) Actin + myosin responsbile for muscle contractions 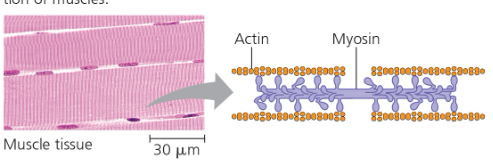 | Defensive proteins- Protects against disease Ex) Antibodies 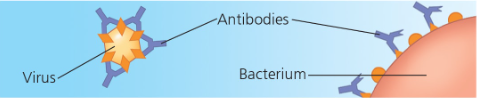 | Transport proteins- transport of substances Ex) Hemoglobin; Transport proteins tansport molecules across membranes (active transport) 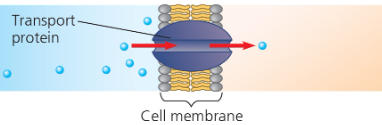 |
Receptor proteins- cell response to chemical stimuli Ex) Receptors in nerve cell membrane detect signaling molecules released by other nerve cells 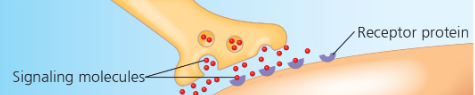 | Structural proteins- support and bind parts together Ex) Collagen + elastin provide a fibrous framework in animal connective tissues 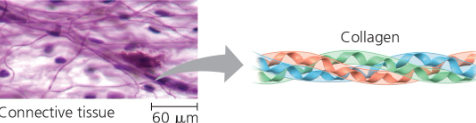 |
Amino Acids
All contain an amino group (NH2) and carboxyl group (COOH) attached to a central alpha (α) carbon
Differs in the side chain/R-group that determine the unique characteristics of the amino acid
Chains of amino acids have a directionality, with an amino acid end (N-terminus) and a carboxyl end (C-terminus)
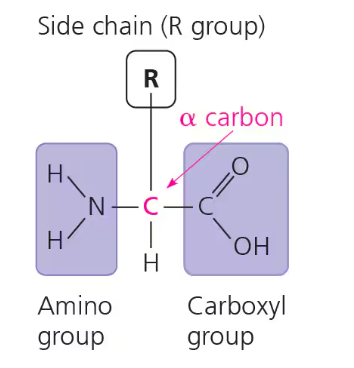
20 total types of amino acids:
Nonpolar R group (hydrophobic)
Hydrocarbon (CHx) on the outside
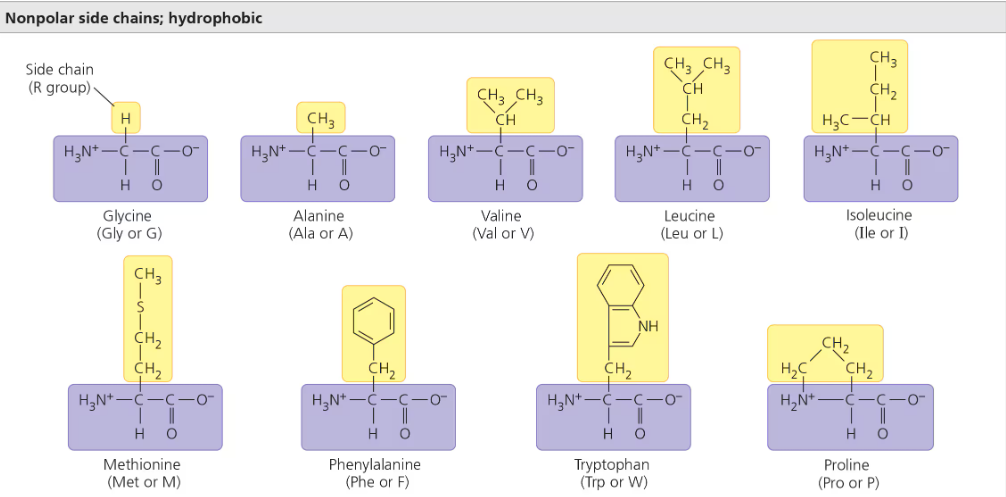
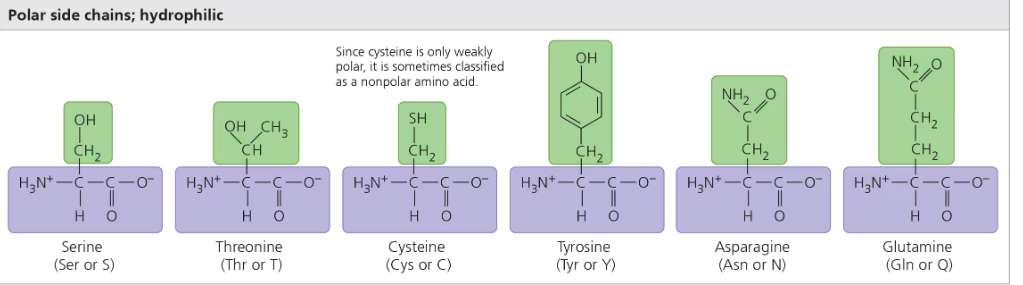
Polar R group (hydrophilic)
Hydroxyl (OH) or animo group (NH2) and Oxygen on the outside
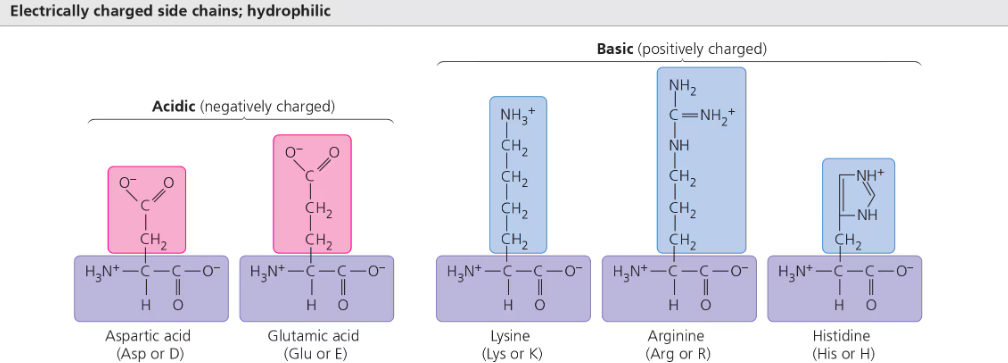
Acidic amino acids have side chains usually negative (-) in charge due to prescence of carboxyl group (COOH) that usually dissociates (ionizes) at cellular pH
Basic amino acids have amino groups (NH2) in side chains generally positive (+) in charge
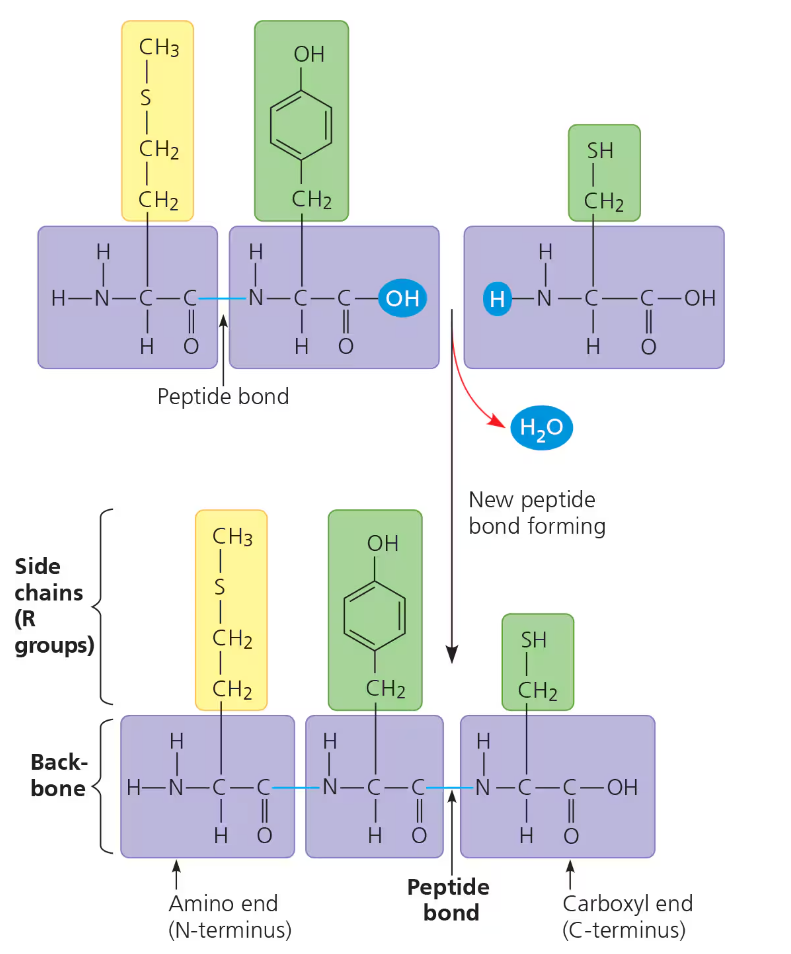
Polypeptides
Polypeptide- a polymer of many amino acids linked by peptide bond formed by dehydration synthesis
Formed between the carboxyl (C-terminus) and amino (N-terminus) groups bond between the C—N molecules → creates the polypeptide backbone
Protein shapes and functions
When a polypeptide is synthesized, the chain may spontaneously fold into different shapes
Globular proteins- spherical shaped; Fibrous proteins- shaped like long fibers
A protein’s structure shapes its function
Ex) Antibodies fit the exact shape of the foreign substance/virus the antibody binds to
Morphine mimics the shape of endorphin binding into receptor proteins on brain cells
Levels of Protein Structure
Primary structure: Linear sequence of amino acids in a protein (polypeptide backbone)
A different arrangement/order of animo acids = polypepide has completely different name/identity
Determines the protein’s shape—where an α helix can form, where β pleated sheets can exist, where disulfide bridges are located, where ionic bonds can form etc.
Secondary structure- held together by hydrogen bonds between the animo (NH2) and carboxyl (COOH) groups of the polypeptide backbone (primary structure)
Take the form of an alpha (α) helix or beta (β) pleated sheet
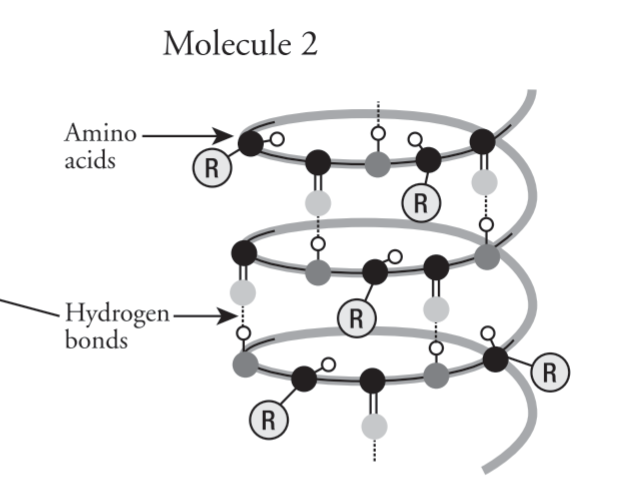
Alpha/(α) Helix
Each transthyrtin polypeptide has only one alpha helix region
Globular proteins have multiple stretches of alpha helixes separated by nonhelical regions (hemoglobin)
Fibrous proteins like alpha keratin have majority alpha helix formation
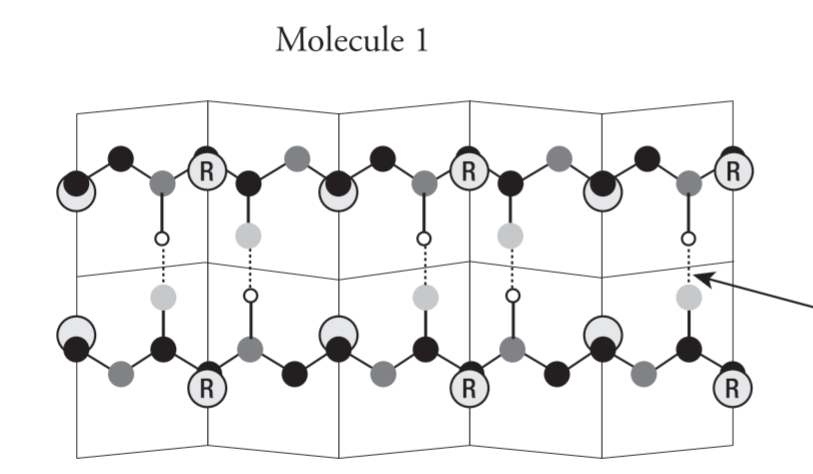
Beta/(β) Pleated Sheet
Make up core for many globular proteins and may dominate some fibrous proteins
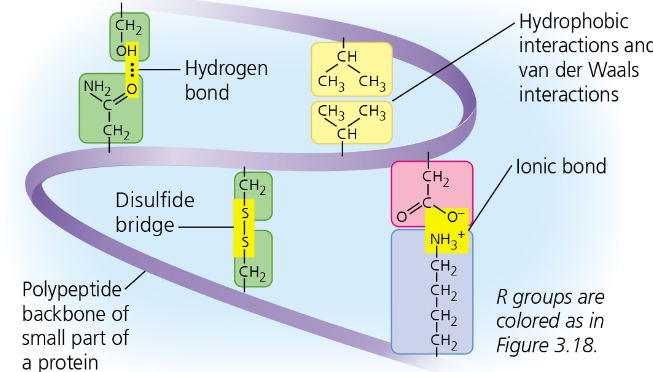
Tertiary Structures: 3D structures stablizied by interactions between R-groups/side chains
4 Types of Interactions:
Hydrophobic -Hydrocarbon (CHx) often clustered on the interior/core of the protein
van der Walls interactions (electric forces between neutral molecules) help hold together nonpolar side chains
Covalent- Disulfite bridges (S2)
Hydrophilic- Hydrogen Bonds (H—O)
Ionic- +/- charged side chains
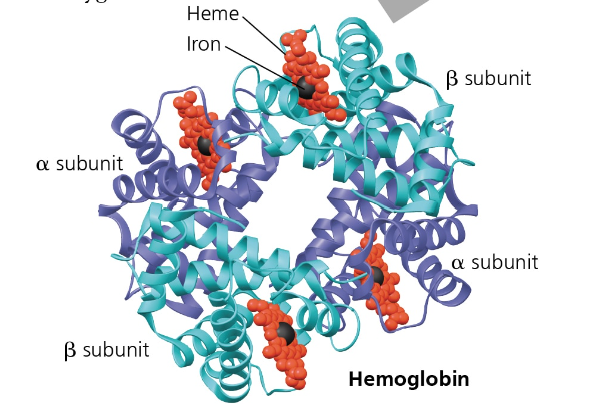
Quartenary Structure- 3D protein structures made of TWO OR MORE polypeptide chains
Ex) Collagen, Hemoglobin
Hemoglobin- carries oxygen on red blood cells to the body
Consists of 4 polypeptide subunits—2 (α) subunits and 2 (β) subunits made primary of alpha helixes
Has a nonpolypeptite component called heme with an iron atom that binds oxygen
Sickle-Cell Disease and change in primary structure:
Caused by substitution of a polar/hydrophilic R-group amino acid (valine) for nonpolar/hydrophobic R-group (glutamic acid) → blood cell misshapen into a sickle shape
Don’t carry as much oxygen; gets stuck in blood vessels; can kill a person at a young age if left untreated
Denaturation- Changes in the shape of a protein
Causes: changes in pH, changes in salinity, high temperatures
Transfer from aqueous environment to a nonpolar solvent (ether/chloroform) → polypeptide chains refold so hydrophobic regions face outward
Chemicals disrupt bonds/interactions (hydrogen, ionic, disulfide bridges)
Nucleic Acids (Textbook ch. 3.6)
Purpose: Store genetic information through 2 types—DNA & RNA
Gene Expression: Includes DNA replication, RNA synthesis, protein synthesis
Molecules: Carbon, Hydrogen, Nitrogen, Oxygen, Phosphate
Nucleic acids are macromolecules—Monomers: nucleotides; Polymers: polynucleotides
Structure of nucleic Acids:
Nucelotides have 3 components—nitrogenous base, five-carbon sugar/pentose, phosphate group
Deoxyribose-DNA; Ribose-RNA
Nitrogenous Bases: Adenine—Thymine(DNA)/Uracil(RNA); Guanine—Cytosine
Pyrimadine: One six-membered ring of carbon and nitrogen atoms
Cytosine, Thymine/Uracil
Purines: Larger than pyrimadines with a six-membered ring fused to a five-member ring
Adenine, Guanine
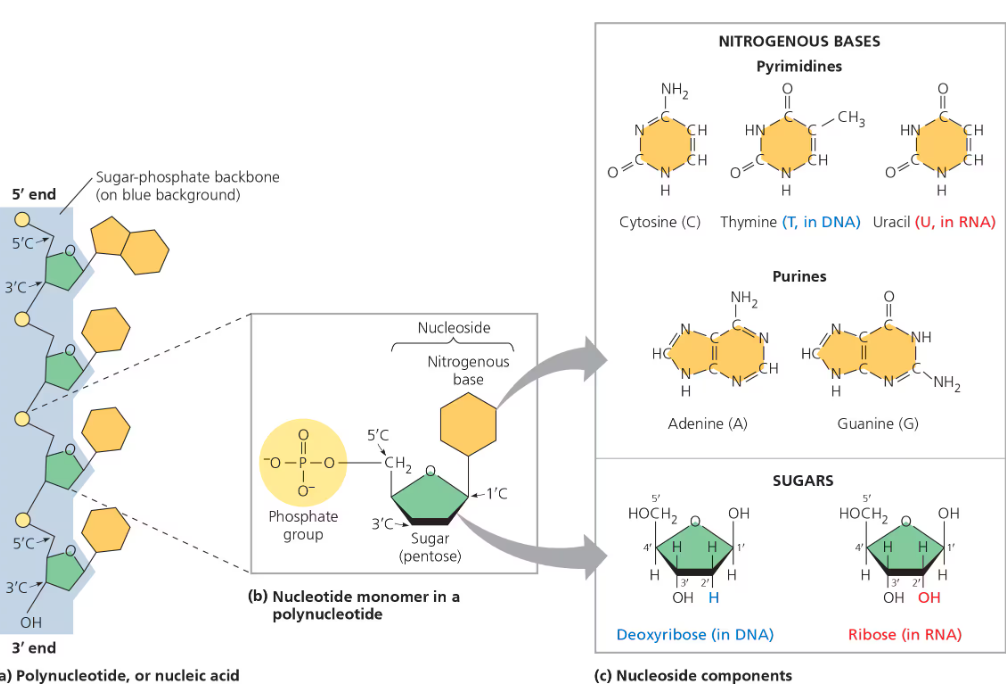
Structure of DNA + RNA
Sugar-phosphate backbone- nucelotides link to one another via dehydration synthesis and joined by a phosphodiester linkage—phosphate group covelently links sugars of 2 nucelotides
Phosphate attached to the 5’ carbon and hydroxyl group on a 3’ carbon end
Directionality 5’ → 3’
Sequence of bases is unique for each gene and provides information for the cell—limitless number of possible sequences ( Ex. 5′-AGGTAACTT-3′)
DNA
Two strands—double helix
Deoxyribose sugar
Antiparallel- sugar-phosphate backbones run in opposite 5’ → 3’ (leading strand) and 3’ → 5’ (lagging strand) directions
Strands held together by hydrogen bonds between the paired nitrogen bases
Adenine—Thymine base pairs have TWO hydrogen bonds; Guanine—Cytosine held together by THREE hydrogen bonds
More hydrogen bonds = more stable the molecule’s structure is
Strands are complementary → able to generate two identical copies of each DNA in a cell preparing to divide
RNA
Ribose sugar
Usually exists as a single strand
Complementary base pairing can occur between regions of 2 RNA molecules/stretches of nucleotides in the same RNA → allows it to take a 3D shape needed for its function
Adenine—Uracil and Guanine—Cytosine base pairs
1.6 Nucleic Acids
DNA vs RNA
Similarities:
Both made from nucleotide monomers/subunits comprise of: 5-carbon sugar, phosphate group, nitrogen group
Each nucleotide connected by covalent bonds forming sugar-phosphate backbone
Each linear srand of nucleotides has a 5’ and 3’ end
Nitrogenous bases perpendicular to sugar-phosphate backbone
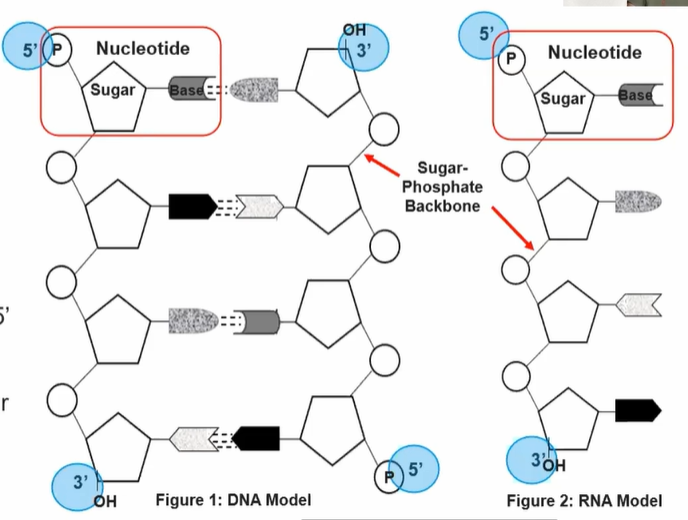
Differences:
Deoxyribose (sugar)

Ribose (sugar)

Nitrogen bases: Thymine, Adenine, Guanine, Cytosine
Nitrogen bases: Uracil, Adenine, Guanine, Cytosine
Double-stranded + antiparallel
Single-stranded
Nucleus Acids
The information storage molecules biological systems
Made of C, H, O, N & P
DNA vs RNA
Deoxyribose
DNA = Adenine, Thymine, Guanine Cytosine
2 Strands
Ribose
RNA = Adenine, Uracil, Guanine, Cytosine
1 Strand
Ribonucleic Acid
Transmits and translates DNA information into protein
Many enzymatic and regulatory functions
1 kind of DNA, -15 types of known RNA at current (3 main types)