Year 12 Unit 3 Chemistry
Reversible Reactions & Equilibrium
Reversible Reactions
Definition: Reversible reactions are chemical reactions that can proceed in both forward and reverse directions, allowing the conversion of reactants to products and vice versa.
Equilibrium Condition: Equilibrium is established in a reversible reaction when the rate of the forward reaction equals the rate of the reverse reaction. At this point, the concentrations of reactants and products remain constant, although both reactions continue to occur.
Steady State System: A steady state can be achieved in a system due to a continual supply of reactants and the removal of products, which allows for constant concentrations in dynamic equilibrium.
Position of Equilibrium: The position of equilibrium indicates whether reactants or products are favored once the system reaches equilibrium.
Example: If the position of equilibrium lies more to the right, products are favored, while a leftward position favors reactants.
Driving Forces of Reactions
Enthalpy (ΔH): Enthalpy is a measure of the heat change during a reaction. A lower or more negative enthalpy value (exothermic reaction) indicates that the reaction is likely to occur spontaneously, whereas a higher value (endothermic reaction) may imply non-spontaneity.
Entropy (ΔS): Entropy reflects the degree of disorder or randomness in a system. A higher or more positive entropy change indicates a greater randomness and generally favors spontaneity in reactions.
Combination of Enthalpy and Entropy: A spontaneous reaction often occurs when the enthalpy is low and entropy is high. Conversely, a reversible reaction is more likely to occur when both enthalpy and entropy are either negative (exothermic and decreased randomness) or positive (endothermic and increased randomness). On the other hand, a non-spontaneous reaction typically occurs when enthalpy is high (requiring energy input) and entropy is low (more ordered state).
Le Chatelier's Principle
Principle: Le Chatelier's Principle states that if a change is applied to a system at equilibrium, the system will adjust in a way that counteracts that change, aiming to restore equilibrium.
Types of Changes affecting Equilibrium:
Concentration Changes:
An increase in reactant concentration shifts the equilibrium position forward, resulting in the production of more products.
A decrease in reactant concentration shifts it backward, consuming reactants to form more products.
Temperature Changes:
Increasing the temperature of an endothermic reaction shifts the equilibrium forward, producing more products.
Conversely, decreasing the temperature favors the reactants in endothermic reactions and the products in exothermic reactions.
Pressure Changes:
An increase in pressure favors the side of the reaction with fewer moles of gas, leading to a lower entropy state.
Equilibrium Constant (Keq)
Definition: The equilibrium constant (Keq) represents the ratio of the concentrations of products to reactants at equilibrium.
Formula:
Keq = ( {[products]}/{[reactants]} )
Equilibrium Scenario Analysis
Scenario Example: Decomposition of Hydrogen Peroxide ( H_2O_2(l) \rightarrow H_2(g) + O_2(g)) with ΔH = +187 kJ
Changes and Predictions:
Increase in [H2]: The forward reaction rate increases, leading to more products.
Decrease in [O2]: This also increases the forward reaction rate.
Decrease Total Pressure: May shift the equilibrium to favor the side with more moles of gas.
Increase Temperature: Forward reaction is favored, resulting in more products produced due to endothermic nature.
Add MnO2 (Catalyst): While this increases the rate of reaction, it does not alter the position of the equilibrium.
Equilibrium Analysis Questions
For complex equilibria involving multiple conditions and reactions, sketch graphs to illustrate the relative concentrations of reactants and products over time. Gain better insights into the dynamic nature of chemical systems.
Entropy Changes Analysis
Predictions of Entropy Changes for Various Reactions:
Reaction A: (2H_2(g) + O_2(g) \rightarrow 2H_2O(g)): Decrease in entropy due to fewer gas moles.
Reaction B: (2SO_3(g) \rightarrow 2S_2(g) + O_2(g)): Increase in entropy, with more gas moles.
Reaction C: (MgCO_3(s) + 2H_3O^+(aq) \rightarrow Mg^{2+}(aq) + 3H_2O(l) + CO_2(g)): Significant increase due to production of gas.
Reaction D: (Ag^+(aq) + Cl^-(aq) \rightarrow AgCl(s)): Decrease in entropy as a solid is formed.
Reaction E: (2C_2H_2(g) + 5O_2(g) \rightarrow 4CO_2(g) + 2H_2O(g)): Decrease in entropy, fewer moles of gas.
Reaction F: (NH_3(g) + HCl(g) \rightarrow NH_4Cl(s)): Decrease in entropy due to formation of solid.
Equilibrium Questions
Keq Predictions: Based on specific conditions, be able to predict how Keq will behave and the resulting outcome at equilibrium.
Effect of Changes: Analyze equilibrium shifts with respect to changes in concentrations, pressure, and temperature, and understand their implications for chemical reactions.
Common Misconceptions in Equilibrium
It is crucial to understand that equilibrium is dynamic and not static; reactions continue in both directions simultaneously.
Adding a substance can shift equilibrium, but it does not inherently alter the value of Keq itself.
Real-world Applications and Experiments
Syringe Experiment with NO Gas: This experiment demonstrates equilibrium through change in color, representing the equilibrium between reactants and products (N2O4(g) + heat ↔ 2NO2(g)).
Controlled Experiments: Investigate how various environmental factors affect equilibrium systems in real chemistry reactions versus controlled laboratory settings.
Summary of Conceptual Understanding
Recognizing the impact of various changes on equilibrium states is vital. Apply Le Chatelier’s Principle effectively in diverse chemical contexts. Develop skills necessary to predict changes in concentrations and equilibrium positions, enhancing the conceptual understanding of dynamic systems within chemical reactions.
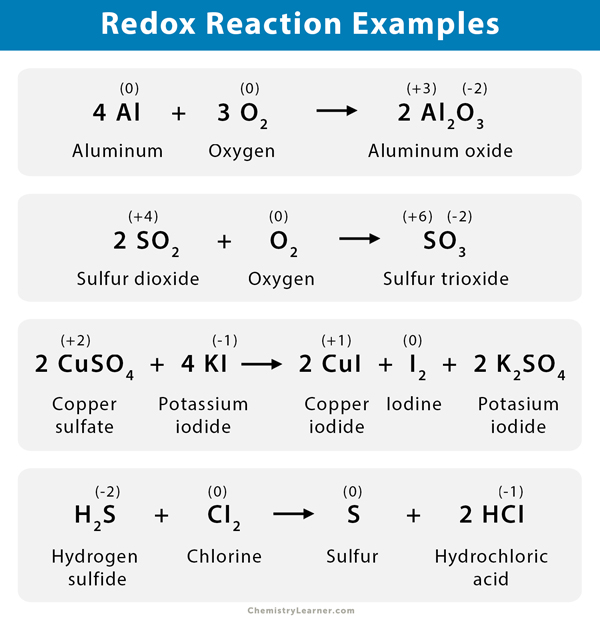
Redox Reactions
Definition
Redox reactions, short for reduction-oxidation reactions, involve the transfer of electrons between chemical species. These reactions are fundamental to energy production and transfer mechanisms in biological systems as well as in various industrial processes.
Components
Oxidation: Defined as the loss of electrons by a chemical species, resulting in an increase in its oxidation state. For example, in the reaction between zinc and copper sulfate (Zn + CuSO4 → ZnSO4 + Cu), zinc is oxidized from a zero oxidation state to +2 as it loses two electrons, while copper is reduced from +2 to zero as it gains those electrons.
Reduction: This is the gain of electrons by a chemical species, leading to a decrease in its oxidation state. Continuing the previous example, copper gains electrons from the zinc and is reduced, demonstrating the interplay of both processes in a redox reaction.
Oxidizing Agent: The oxidizing agent gains electrons and is reduced itself. In the example of zinc and copper sulfate, copper sulfate acts as the oxidizing agent. It facilitates the oxidation of zinc, allowing electrons to flow from zinc to copper.
Reducing Agent: The reducing agent loses electrons and is oxidized in the process. Zinc, in this example, serves as the reducing agent as it donates electrons to copper while being oxidized to Zn²⁺.
Half-Reactions: Each redox reaction can be dissected into two half-reactions. For the above example, the oxidation half-reaction can be represented as Zn → Zn²⁺ + 2e⁻, and the reduction half-reaction can be represented as Cu²⁺ + 2e⁻ → Cu. This separation allows easier tracking of electron transfer.
Balancing Redox Reactions
Balancing redox reactions ensures the conservation of mass and charge.
Half-Reaction Method: This involves balancing each half-reaction separately. Consider the reaction between hydrogen and fluorine:
Oxidation half-reaction: 2H₂ → 4H⁺ + 4e⁻
Reduction half-reaction: F₂ + 4e⁻ → 2F⁻By ensuring equal numbers of electrons are exchanged, and ultimately combining these half-reactions leads to the balanced equation H₂ + F₂ → 2HF.
Redox Titration: This quantitative technique determines the concentration of an unknown solution by reacting it with a titrant of known concentration. For instance, in determining the concentration of iron(II) ions using potassium permanganate (KMnO₄), the redox reaction changes the Mn from +7 to +2 as it accepts electrons, allowing precise calculations of the iron’s concentration.
Equilibrium
Definition
Equilibrium in a chemical reaction refers to the state where the concentrations of reactants and products remain constant over time. This dynamic state occurs as both forward and reverse reactions happen simultaneously at equal rates.
Dynamic Nature
Equilibrium does not imply equal concentrations of reactants and products; rather, it indicates that the rates of formation of products and reactants are equal. For example, in the synthesis of ammonia (N₂ + 3H₂ ⇌ 2NH₃), at equilibrium, there might be higher concentrations of products (NH₃) relative to reactants (N₂ and H₂), yet the rates of formation stay equal.
Le Chatelier's Principle
According to Le Chatelier's Principle, if an external change (such as pressure, concentration, or temperature) is applied to a system at equilibrium, the system will respond to counteract that change by shifting the equilibrium position to the side that alleviates the change. For instance, increasing the concentration of reactants in the ammonia synthesis will shift the equilibrium to favor product formation, producing more NH₃.
Equilibrium Constant (K)
The equilibrium constant (K) quantitatively expresses the ratio of concentrations of products to reactants at equilibrium. For the ammonia reaction, the equilibrium constant expression is K = [NH₃]² / ([N₂][H₂]³).
K > 1: Indicates that, at equilibrium, the concentration of products is favored (e.g., reactions like the synthesis of strong acids such as HCl).
K < 1: Suggests that the reactants are favored at equilibrium, such as in the formation of solid precipitates where little product forms.
Factors Affecting Equilibrium
Several factors can influence the position of equilibrium:
Concentration Changes: Increasing the concentration of reactants shifts equilibrium towards products, leading to more product formation.
Temperature Changes: In an exothermic reaction, increasing temperature shifts equilibrium towards reactants, while in an endothermic reaction, temperature increase favors product formation.
Pressure Changes: Changes in pressure affect gaseous reactions by shifting the equilibrium towards the side with fewer moles of gas. For instance, in the reaction 2CO(g) + O₂(g) ⇌ 2CO₂(g), increasing pressure favors the formation of CO₂ due
Single Displacement Reactions
Definition
Single displacement reactions (also called single replacement reactions) occur when one element in a compound is replaced by another element.
General Equation
The general form of a single displacement reaction can be represented as:
A + BC → AC + B
Where A is a free element displacing B from the compound BC.
Characteristics
Typically occurs with metals or halogens.
A more reactive element displaces a less reactive element from its compound.
Examples of Single Displacement Reactions
Equation Description | |
[Zn + CuSO_4 → ZnSO_4 + Cu] | Zinc displaces copper from copper sulfate. |
[2Na + 2H_2O → 2NaOH + H_2] | Sodium displaces hydrogen from water. |
[Cl_2 + 2KBr → 2KCl + Br_2] | Chlorine displaces bromine from potassium bromide. |
[Fe + CuCl_2 → FeCl_2 + Cu] | Iron displaces copper from copper(II) chloride. |
[Mg + 2HCl → MgCl_2 + H_2] | Magnesium displaces hydrogen from hydrochloric acid. |
How Redox Reactions Work
Single displacement reactions are often redox reactions, which involve the transfer of electrons between species, resulting in changes in oxidation states. In these reactions:
Oxidation occurs when the element that displaces another (A) loses electrons and its oxidation state increases.
Reduction occurs when the displaced element (B) gains electrons, leading to a decrease in its oxidation state.
For example, in the reaction of zinc with copper(II) sulfate: [ Zn + CuSO_4 → ZnSO_4 + Cu ]
Zinc (Zn) is oxidized from an oxidation state of 0 to +2 as it loses two electrons: [ Zn → Zn^{2+} + 2e^{-} ]
Copper (Cu) is reduced from an oxidation state of +2 to 0 as it gains those electrons: [ Cu^{2+} + 2e^{-} → Cu ] Thus, the reaction involves both oxidation (of zinc) and reduction (of copper), a hallmark of redox processes.
Observations
Formation of a new compound.
Release of a gas (like H₂ or Cl₂).
Color change or formation of precipitate may occur depending on the reactants.
Conclusion
Single displacement reactions are essential in various chemical processes including metal extraction, reactions in batteries, and more. Understanding their mechanism allows chemists to predict reactivity and outcomes. Furthermore, recognizing the redox nature of these reactions aids in comprehending the underlying electron transfer processes.