Chapter 8: An Introduction to Metabolism
8.1 An Organism’s Metabolism Transforms Matter and Energy
Metabolic pathway: a specific molecule is altered in a series of defined steps, resulting in a certain product.
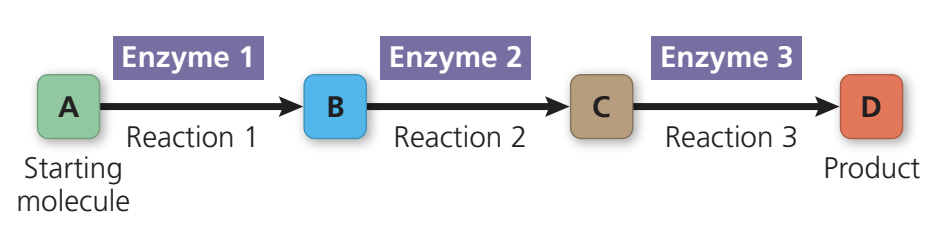
Bioenergetics: study of energy flow through living organisms.
Energy: capacity to cause change, do work by moving matter against opposing forces.
Energy exists in various forms; cells transform energy.
Kinetic energy: energy associated with motion. Moving objects perform work by imparting motion.
Thermal energy: kinetic energy from random atom/molecule movement; heat is thermal energy in transfer.
Light: energy harnessed to perform work (e.g., photosynthesis).
Potential energy: energy matter possesses due to location/structure.
Chemical energy: potential energy available for release in reactions. Catabolic pathways release energy by breaking down complex molecules.
Complex molecules (e.g., glucose): high in chemical energy.
Catabolic reactions: break bonds, form others, releasing energy, resulting in lower-energy breakdown products.
Biochemical pathways: cells release chemical energy from food, use it to power life.
Energy transformations:
Climbing: kinetic energy of muscle movement -> potential energy.
Diving: potential energy -> kinetic energy.
Food chemical energy -> kinetic energy, heat, CO_2, H_2O.
Thermodynamics: studies energy transformations in matter; system = matter under study, surroundings = everything else.
Isolated system: cannot exchange energy/matter with surroundings.
Open system: can transfer energy/matter. Organisms are open systems.
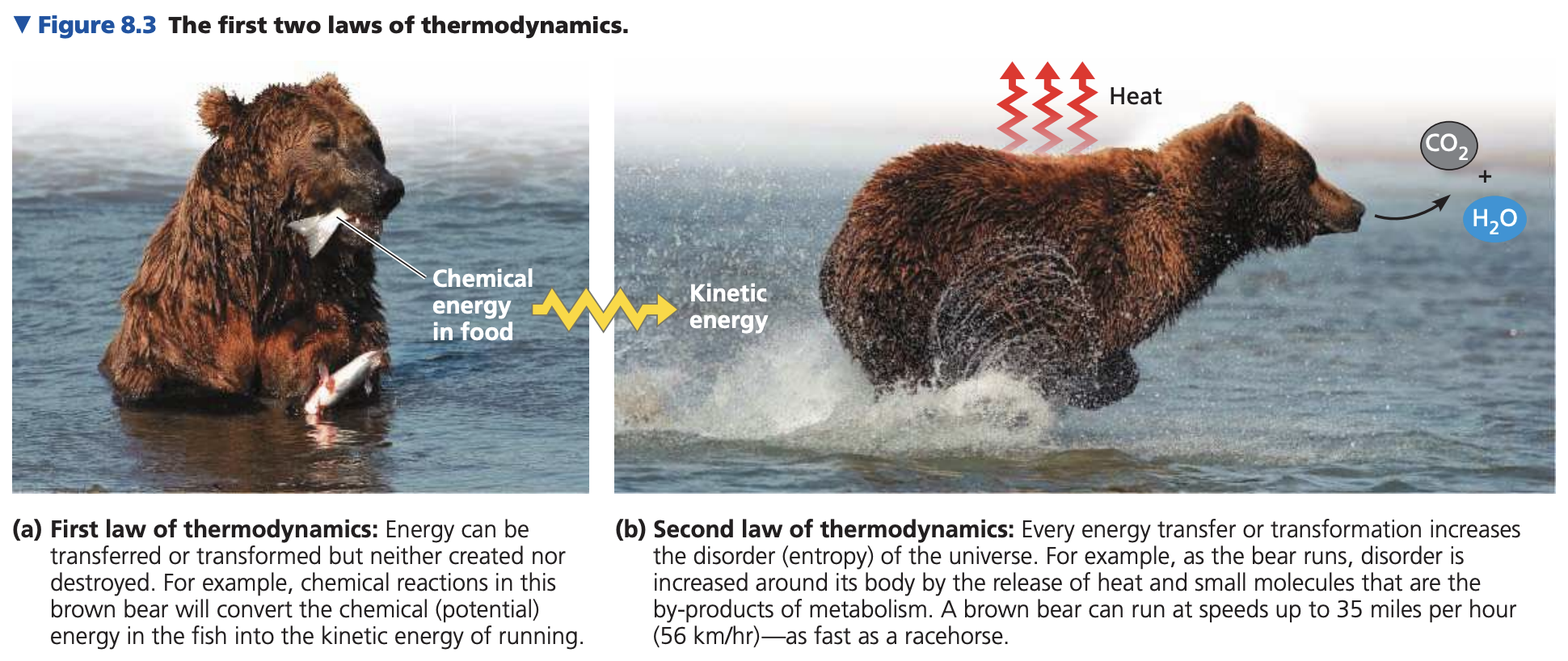
First law of thermodynamics: energy is constant; transferred/transformed, not created/destroyed (conservation).
Plants: sunlight -> chemical energy.
Brown bears: food chemical energy -> kinetic/other energy.
Second law of thermodynamics: every energy transfer/transformation increases entropy (disorder).
Energy transformation: some energy becomes thermal, released as heat.
System uses thermal energy only with temperature difference.
Entropy: molecular disorder/randomness.
Spontaneous processes: increase entropy, occur without energy input (energetically favorable).
Nonspontaneous processes: decrease entropy, require energy input.
Living systems increase entropy of surroundings.
Organisms: ordered matter/energy in, less ordered forms out.
Energy: light in, heat out.
Complex organisms evolved from simpler ones; entropy of a particular system may decrease if the total entropy of the universe increases.
8.2 The Free-Energy Change of a Reaction Tells Us Whether or Not the Reaction Occurs Spontaneously
Gibbs free energy (G): energy that can perform work when temperature/pressure are uniform.
Change in free energy (\DeltaG): \DeltaG = \DeltaH - T\DeltaS, \DeltaH = enthalpy (total energy), \DeltaS = entropy change, T = absolute temperature (Kelvin).
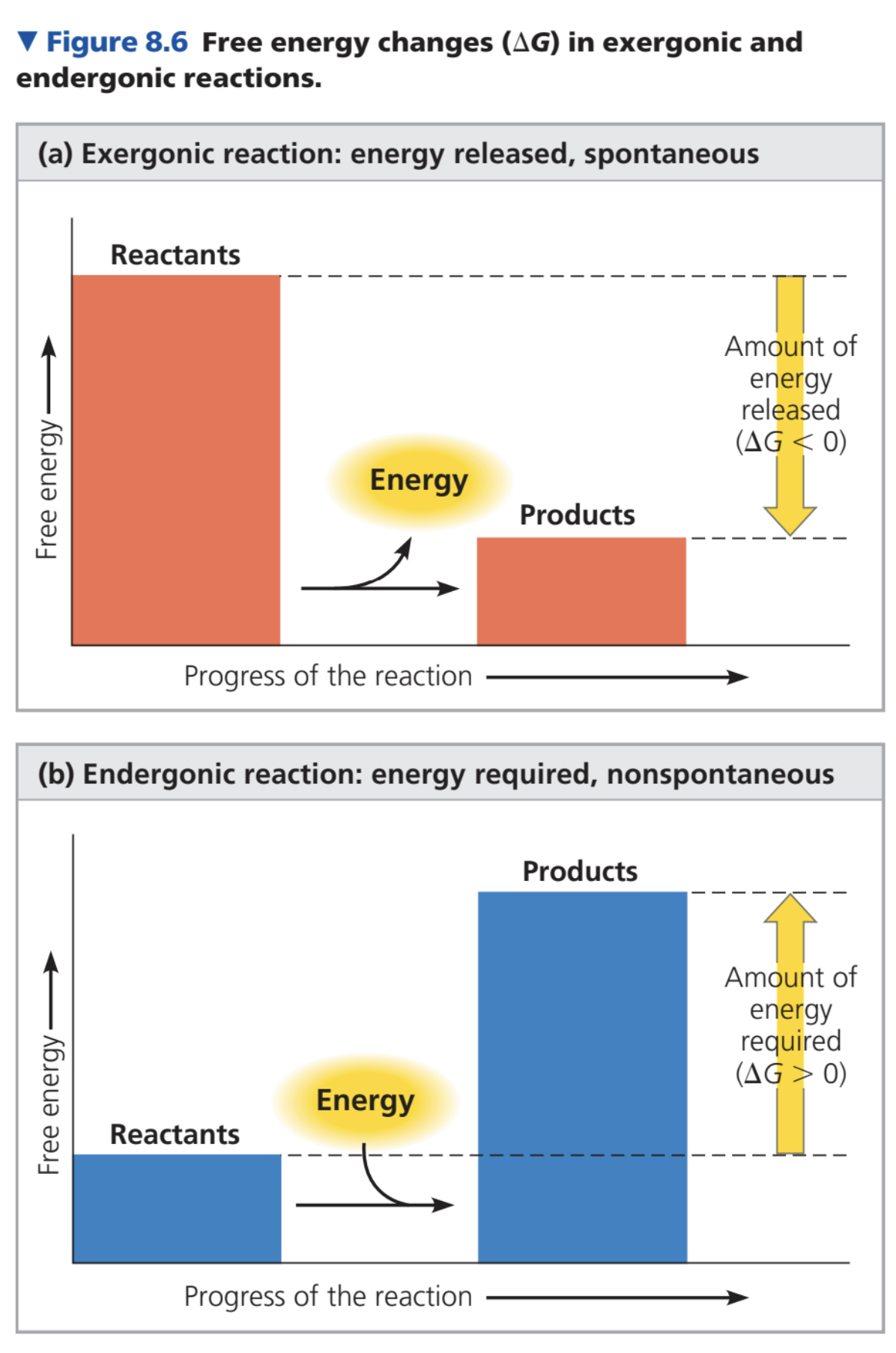
Processes with negative \DeltaG are spontaneous; decrease system’s free energy.
Free energy: measure of system’s instability.
Unstable systems (higher G) change to more stable (lower G).
Equilibrium: state of maximum stability; forward/reverse reactions at same rate.
At equilibrium: G is lowest, system can do no work.
Process is spontaneous, can perform work only moving toward equilibrium.
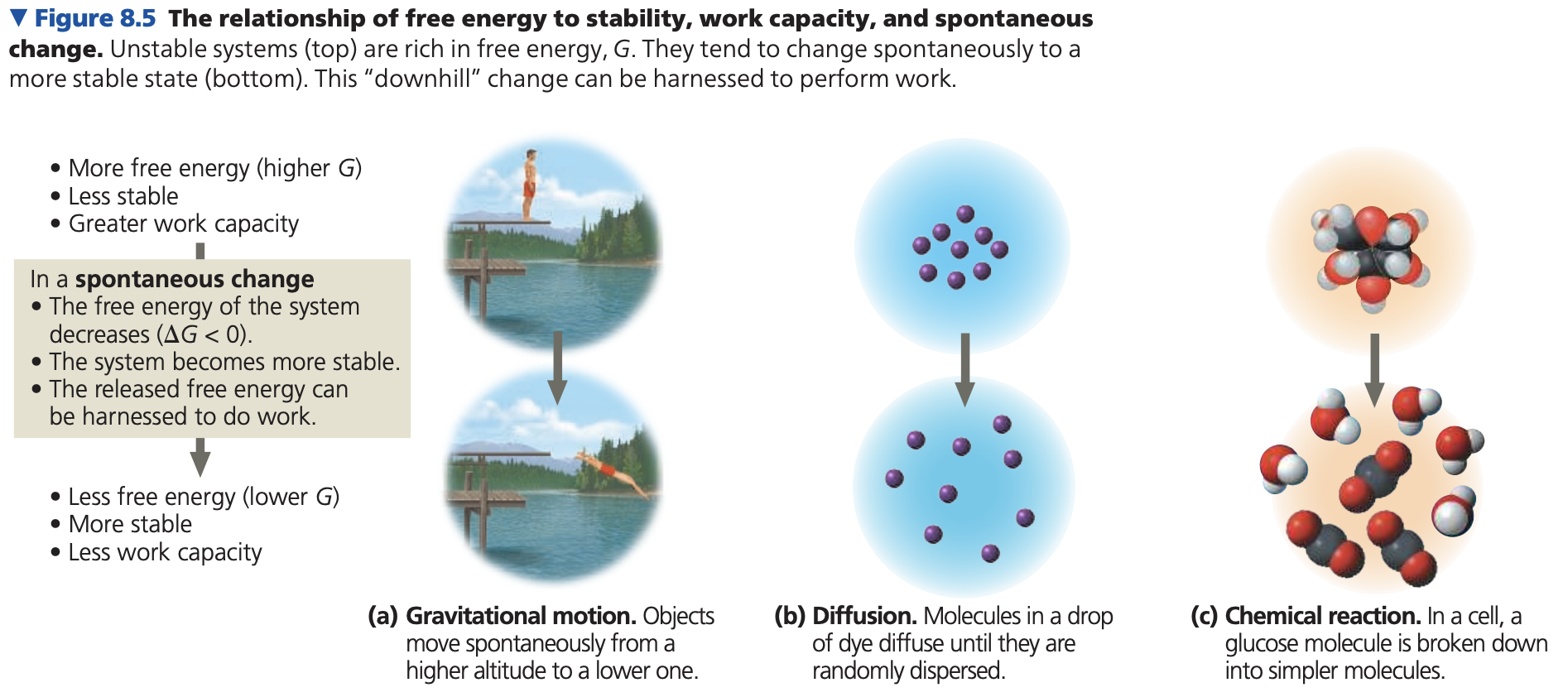
Exergonic reactions: release energy; \DeltaG negative, spontaneous.
Endergonic reactions: absorb energy; \DeltaG positive, non-spontaneous.
Breaking bonds: requires energy.
“Energy stored in bonds”: potential energy released when new bonds form after original bonds break if products have lower free energy than reactants.
Reactions in isolated system: reach equilibrium, can do no work.
Metabolism chemical reactions are reversible, respiration reactions pulled in one direction, kept out of equilibrium.
Maintaining lack of equilibrium: reaction product doesn't accumulate, becomes reactant in next step; waste expelled.
Overall reaction sequence: kept going by free-energy difference between glucose and O_2 at the top of the energy hill and CO_2 and H_2O at the “downhill” end.
8.3 ATP Powers Cellular Work by Coupling Exergonic Reactions to Endergonic Reactions
Cells do chemical, transport, and mechanical work.
Energy coupling: exergonic process drives endergonic one.
ATP (adenosine triphosphate): mediates most energy coupling, immediate energy source.
ATP contains ribose, adenine, triphosphate group.
Hydrolysis of ATP releases energy:
ATP + H_2O-> ADP + Pi, \DeltaG = -7.3 kcal/mol (standard conditions).
Terminal phosphate bond broken by water.
Actual \DeltaG under cellular conditions: ~ -13 kcal/mol.
ATP phosphate bonds: not unusually strong; release comes from chemical change to lower free energy.
ATP hydrolysis drives chemical reactions that are endergonic by forming a phosphorylated intermediate
Transport and mechanical work powered by ATP hydrolysis, changing protein shape by phosphorylation
ATP is renewable: regenerated by adding phosphate to ADP, using energy from exergonic breakdown (catabolism).
ATP cycle: shuttling inorganic phosphate and energy, coupling energy-yielding (exergonic) to energy-consuming (endergonic) processes.
ADP + Pi -> ATP + H2O, \DeltaG = + 7.3 kcal/mol (standard conditions)
8.4 Enzymes Speed Up Metabolic Reactions by Lowering Energy Barriers
Enzymes: macromolecules act as catalysts, speeding up reactions without being consumed.
Enzymes catalyze reactions by lowering the free energy of activation (E_A).
They cannot change the \DeltaG for a reaction nor make an endergonic reaction exergonic.
Enzymes are very specific for the reactions they catalyze.
The reactant that the enzyme acts on is called the substrate.
The substrate binds to the active site, forming an enzyme-substrate complex.
Enzyme + Substrate(s) -> Enzyme-substrate complex -> Enzyme + Product(s).
Active site: pocket/groove on enzyme surface.
Substrate enters active site, enzyme changes shape slightly due to interactions. This is called induced fit.
In enzymatic reactions:
Active site: template for substrates to come together in orientation.
They may stretch the substrate molecules toward their transition state form, stressing and bending critical chemical bonds.
Active site may provide a microenvironment that is more conducive to a certain reaction.
Amino acids in the active site may directly participate in the chemical reaction by brief covalent bonding between the substrate and the side chain of an amino acid of the enzyme.
Substrate concentration high enough: all enzyme molecules have active sites engaged (saturated).
Cells increase reaction rate by producing more enzyme molecules.
Enzyme activity affected by temperature/pH:
Each enzyme has optimal temperature/pH for greatest reaction rate.
Cofactors: nonprotein helpers required for catalytic activity; inorganic (metal atoms) or organic (coenzyme).
Most vitamins act as coenzymes or raw materials.
Enzyme inhibitors: chemicals selectively inhibit enzyme action:
Competitive inhibitors: resemble substrate, compete for active site.
Noncompetitive inhibitors: bind elsewhere, change enzyme shape.
Mutations can result in proteins with changed amino acids and environmental conditions where the new function benefits the organism. Natural selection would tend to favor the mutated form of the gene.
8.5 Regulation of Enzyme Activity Helps Control Metabolism
Cells regulate metabolic pathways by switching on and off the genes that encode specific enzymes or by regulating the activity of enzymes once they are made.
Allosteric regulation: protein’s function at one site affected by regulatory molecule binding to separate site.
Allosteric regulation: inhibition or stimulation of enzyme activity.
Allosteric regulation: activating/inhibiting regulatory molecule binds to allosteric site.
Regulator concentrations can cause sophisticated patterns in enzyme activity.
Some allosteric enzymes: substrate binding to one active site in multisubunit enzyme triggers shape change in all subunits, increasing catalytic activity (cooperativity).
Feedback inhibition: metabolic pathway halted by end product inhibiting enzyme early in pathway.
Cellular structures help order metabolic pathways by localizing enzymes. Enzyme teams can form multienzyme complexes.