Topic 2 - Chemical bonding and structure
Crystal lattice structure
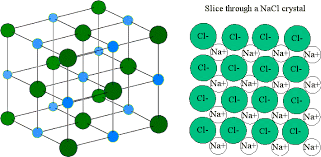
In an ionic bond there are alternating positive and negative ions
Ionic bonds have strong electrostatic attraction, they are conductive when molten or aqueous, they have high melting and boiling points, are solids at room temp and are soluble in water as the charged ions can interact with the polar water molecules
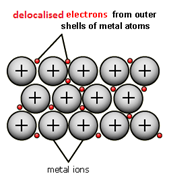
Metallic bonding consists of cations surrounded by delocalised electrons
Metallic bonds have strong electrostatic attraction, high conductivity as they have delocalised electrons that can move freely, they are solid at room temperature and have a high melting and boiling point. They are insoluble in water as the metallic bonds are too strong
Simple covalent bonds are individual molecules held together by weak intermolecular forces
They have low melting and boiling points, are not conductive and are liquid or gas at room temperature. Some of them can dissolve in water but only ones with polar bonds.
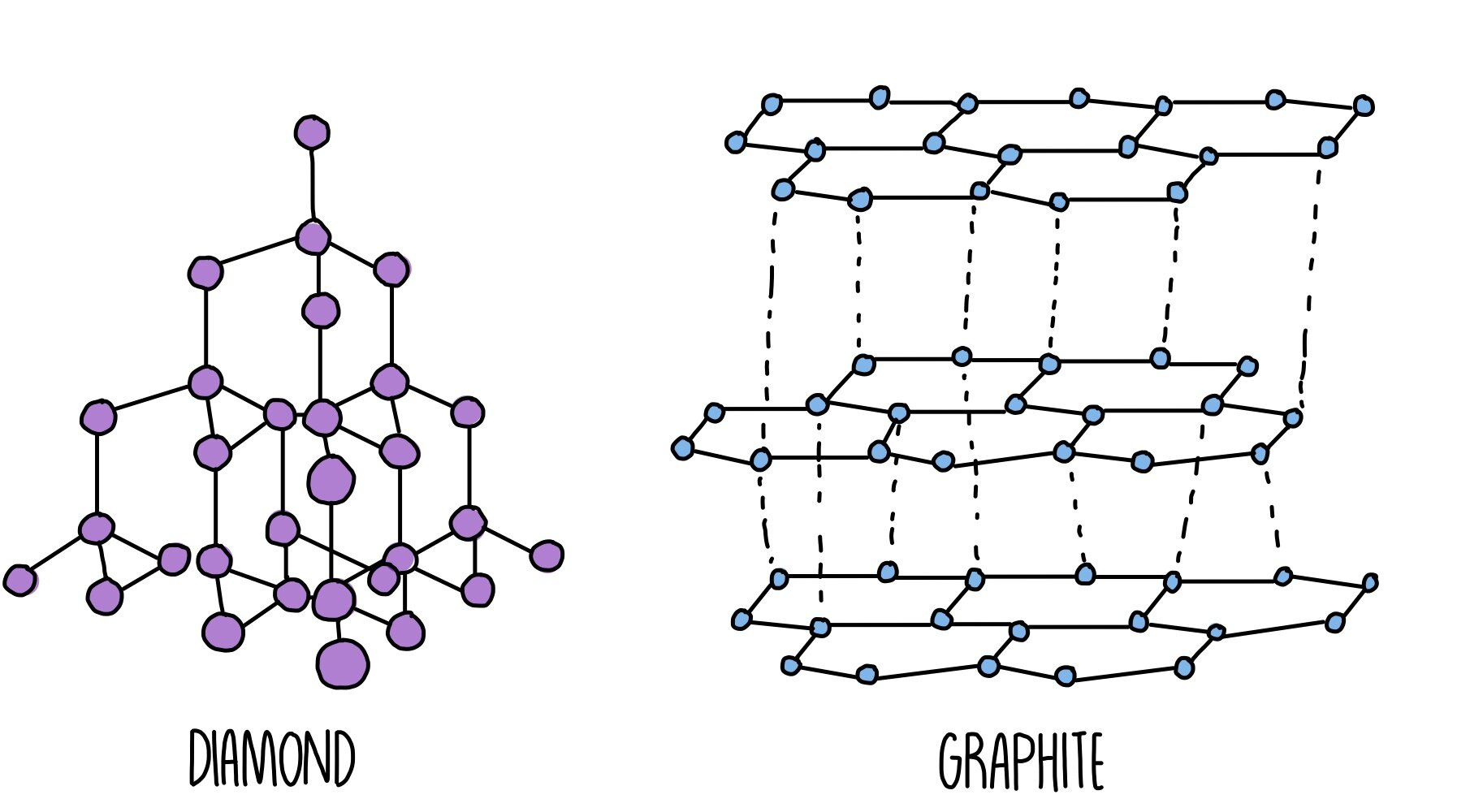
Giant covalent molecules are held together by covalent bonds
Covalent bonds are very strong so they have very high melting and boiling points making them solid at room temperature. Some of them can carry charge but it is dependant on delocalised electrons. The strong covalent bonds prevent dissolution.
Ionic bonding
An ionic bond is the electrostatic attraction between oppositely charged ions
Cation = lose electrons to become a positive ion (usually a metal)
Anion = gain electrons to become negative ions
The ionic radius is a measure of the size of an ion. The size increases with negative charge and decreases with positive charge. It decreases down a group due to an increasing number of electron shells and greater shielding. Across a period it decreases for cations but increases for anions.
For cations as more protons are present the electrostatic attraction increases and draws the electrons closer. For anions the addition of electrons results in more shielding.
Covalent bonding
A covalent bond is strong electrostatic attraction formed between a shared pair of electrons and the nuclei of the bonded atoms.
One type of covalent bonding is dative bonding which is where all the electrons involved in forming the covalent bond come from the same atom. IT IS ALSO KNOWN AS COORDINATE BONDING
Bond energy → amount of energy required to break one mole of a covalent bond in the gaseous state.
Shapes of molecules
Lone pairs cause more repulsion than bonding pairs
Electrons will arrange themselves as far away as possible to minimise repulsion
Lone pairs | Bond pairs | Shape | Angle |
None | 2 | Linear | 180 |
None | 3 | Trigonal planar | 120 |
None | 4 | Tetrahedral | 109.5 |
None | 5 | Trigonal bipyramidal | 120 + 90 |
None | 6 | Octahedral | 90 |
2 | 2 | V-shaped | 104.5 |
1 | 3 | Triangular pyramidal | 107 |
Polar bonds
Electronegativity is the measure of an atoms ability to attract the shared pair e=of electrons in a covalent bond towards itself. The higher number on the scale means it is more electronegative.
Across a period the electronegativity increases as does nuclear charge however atomic radius decreases
Down a group the electronegativity decreases but both the nuclear charge and atomic radius increase
Polar bonds are formed from differences in electronegativity. Bonding electrons are more strongly attracted to the more electronegative atom and this unequal sharing makes the bond polar. Polar bonds have a permanent electric dipole (separation of positive and negative charges).
We can use the difference in electronegativities to work out what type of bond to atoms would have
Difference in electronegativity | Type of bond |
less than 1 | non-polar covalent |
1-2 | polar covalent |
2+ | ionic |
Intermolecular forces
Intermolecular forces are weak attractions between molecules
There are three types of intermolecular force: London forces (also known as dipole-induced-dipole) which are found between all molecules. Dipole-dipole forces which are slightly stronger and are found with polar molecules. Hydrogen bonds are the strongest type of intermolecular force but they form with only polar molecules with hydrogen bonded to fluorine, oxygen, or nitrogen
London
London forces arise due to temporary fluctuations in electron distribution around atoms. Electrons are constantly moving, if there are more electrons on one side of the atom than the other then a temporary dipole will be created. The temporary dipole can induce opposite dipole in nearby atom, which means there is weak attraction between atoms
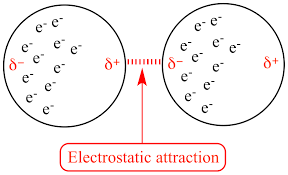
Larger atoms have more electrons so will form stronger temporary dipoles. The stronger the London forces are the high the boiling point will be
Permanent dipole-dipole
These arise from unequal sharing to electrons in covalent bonds and is the electrostatic attraction between partial positive and partial negative charges.
Permanent dipole-dipole forces are in addition to and not instead of London forces, this consequently causes high boiling points

Hydrogen bonds
Hydrogen bonds occur when hydrogen is bonded to highly electronegative elements (fluorine, oxygen, nitrogen) these bonds are highly polar due to large electronegativity differences.
Substances that form hydrogen bonds are usually soluble in water, and have high melting and boiling points.
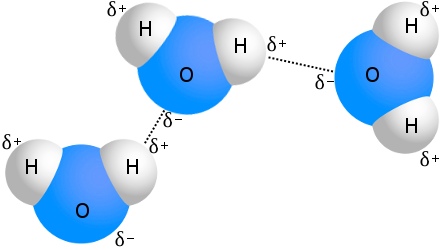
Solutions and solubility
There are two types of solvents, polar and non-polar. Polar solvents are made of polar molecules and non-polar solvents are made of non-polar molecules
Ionic compounds will dissolved in polar solvents such as water as they have both positive and negative ions that are attracted to oppositely charged ends of the water molecule and then the water molecule can surround the ionic compound. Alcohols will dissolve in water as they have the ability to form hydrogen bonds however the solubility will decrease as the number of carbon atoms increases.
If the dipoles aren’t strong enough to form hydrogen bonds then some polar molecules can’t dissolve in water.
Non-polar substances will dissolve in non-polar solvents.
Giant covalent structures
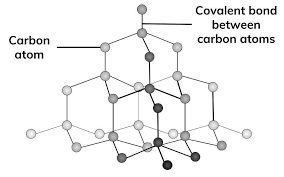
Diamond has very strong covalent bonds holding it together so is very hard, this also means it has a high melting point.
It is a good thermal conductor however cannot conduct electricity as it does not have delocalised electrons to carry charge.
It is insoluble due to the strength of the covalent bonds
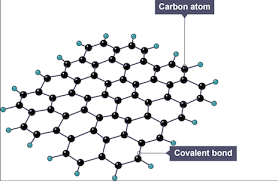
Graphene is a single layer of carbon atoms with a the same structure as graphite, there are 3 bonds per carbon atom meaning there are delocalised electrons which move through the structure carrying charge.
Graphene is very strong
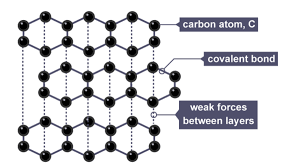
Graphite also has three bonds per carbon atom leading to delocalised electrons that can carry charge. There are strong covalent bonds between carbon atoms in the same layer however connecting the layers are weak intermolecular forces making it slippery. The intermolecular forces also mean graphite has a lower melting point.
Metallic bonding
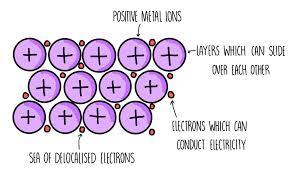
Metallic bonding is the electrostatic attraction between positively charged metal ions and negatively charged delocalised electrons.
The strength of the metallic bond is determined by the number of delocalised electrons, charge of the cation and the radius of the cation