Chapter 17 Class Notes
Pyruvate Dehydrogenase and the Citric Acid Cycle
Section 17.1 The Citric Acid Cycle
Harvests High-Energy Electrons
citric acid cycle (CAC) = series of oxidation–reduction reactions that result in the oxidation of an acetyl group to two molecules of CO2
the final pathway for the oxidation of fuel molecules
oxidation generates high-energy electrons used to power ATP synthesis
important sources of precursors for biosynthesis
also called the tricarboxylic acid (TCA) cycle or Krebs cycleAcetyl CoA
Most fuel molecules enter the citric acid cycle as acetyl CoA (acetyl coenzyme A).
entry way to citric acid cycle
ADP with linker group from pyruvate, suck away all the electrons possible
The Pyruvate Dehydrogenase Complex and the Citric Acid Cycle
pyruvate dehydrogenase complex = a large enzyme complex that oxidatively decarboxylates pyruvate to acetyl CoA under aerobic conditions
rest of C’s go to CO2
Acetyl CoA enters the citric acid cycle where all remaining carbons are completely oxidized to CO2.
Reactions of the pyruvate dehydrogenase complex and the citric acid cycle take place in the mitochondrial matrix.
Mitochondria Have Distinct Compartments Defined by Two Membranes
An Overview of the Citric Acid Cycle
The citric acid cycle removes electrons from acetyl CoA and uses these electrons to reduce NAD+ and FAD to form NADH and FADH2.
drop off electrons and then transport them to oxidative phosphorylation
The Electron-Transport Chain
electron-transport chain = a series of membrane proteins that electrons released in the re-oxidation of NADH and FADH2 flow through to generate a proton gradient across the inner mitochondrial membrane
Protons flow through ATP synthase to generate ATP from ADP and inorganic phosphate.
Cellular Respiration Removes High-Energy Electrons from Carbon Fuel Molecules to Generate ATP
Section 17.2 The Pyruvate Dehydrogenase Complex Links Glycolysis to the Citric Acid Cycle
The pyruvate dehydrogenase complex:
is a highly integrated unit of three distinct enzymes in the mitochondrial matrix.
oxidatively decarboxylates pyruvate to acetyl CoA.
The Pyruvate Dehydrogenase Complex Connects Glycolysis to the Citric Acid Cycle
The reaction catalyzed by the pyruvate dehydrogenase complex is an irreversible link between glycolysis and the citric acid cycle.
fats can’t be used to make glucose in gluconeogenesis
enter as acetyl CoA, can’t go back to pyruvate because pyruvate to acetyl CoA is irreversible
Pyruvate Dehydrogenase Complex of E. coli
TABLE 17.1 Pyruvate dehydrogenase complex of E. coli
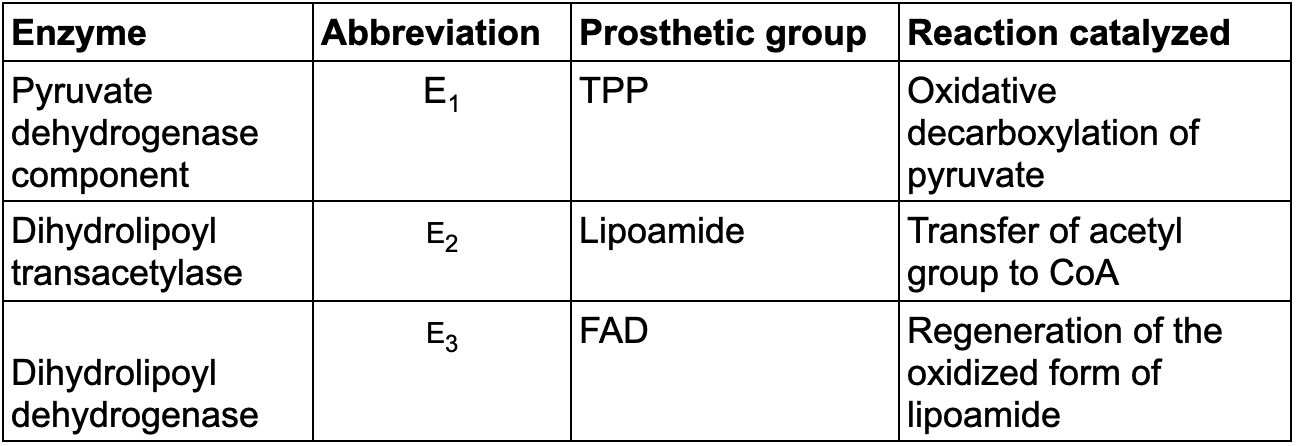
Enzyme Abbreviation Prosthetic group Reaction catalyzed Mechanism: The Synthesis of Acetyl Coenzyme A from Pyruvate Requires Three Enzymes and Five Coenzymes
The catalytic cofactors are thiamine pyrophosphate(TPP), lipoic acid, and FAD.
TPP: thiamine pyrophosphate
be able to recognize structures
The stochiometric cofactors (cofactors that function as substrates) are CoA and NAD+
The Conversion of Pyruvate into Acetyl CoA Consists of Three Steps, Plus a Regeneration Step
Steps must be coupled because the free energy from the decarboxylation step drives the formation of NADH and acetyl CoA.
The Decarboxylation Step
Pyruvate combines with the coenzyme TPP and is decarboxylated to yield hydroxyethyl-TPP.
the rate-limiting step in acetyl CoA synthesis
catalyzed by the pyruvate dehydrogenase component (E1) of the multienzyme complex
TPP is the prosthetic group of E1
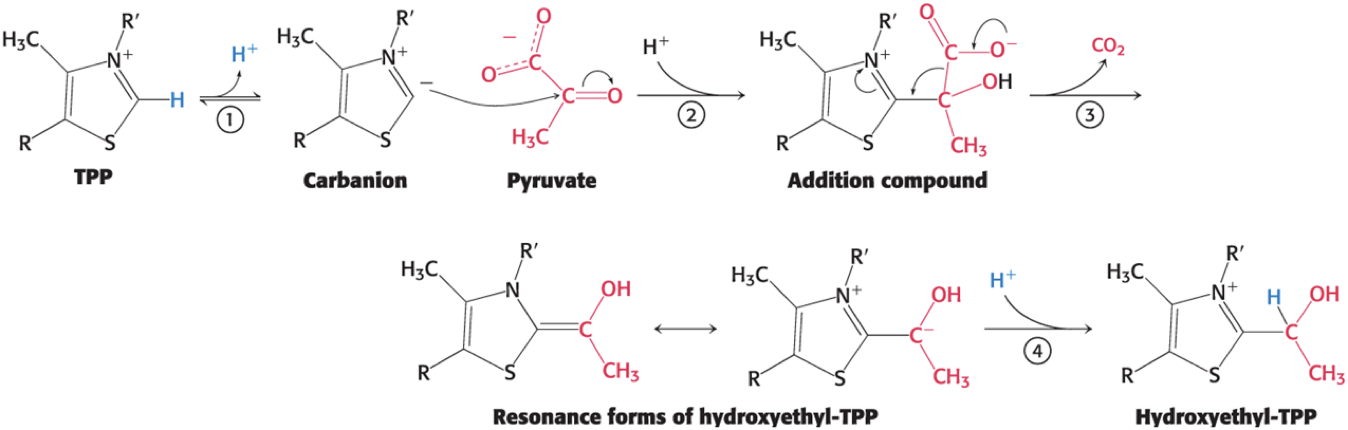
The Mechanism of the Decarboxylation Step
Step 1: the carbon center of TPP ionizes to form a carbanion
Step 2: the carbanion readily adds to the carbonyl group of pyruvate
Step 3: decarboxylation of pyruvate
the positive charged ring of TPP stabilizes the negative charge resulting from the decarboxylation
Step 4: protonation yields hydroxyethyl-TPP
The Mechanism of the E1 Decarboxylation Reactions Uses a Critical Thiamine-Derived Prosthetic Group
The Oxidation Step
lipoamide = a derivative of lipoic acid that is linked to the side chain of a Lys residue by an amide linkage
the hydroxyethyl group attached to TPP oxidizes to form an acetyl group while being simultaneously transferred to lipoamide, yielding acetyllipoamide
forms an energy-rich thioester bond
the disulfide group of lipoamide is reduced
catalyzed by E1
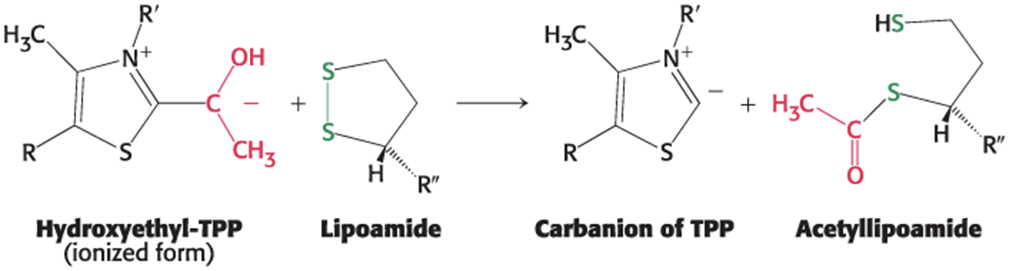
The Formation of Acetyl CoA Step
The acetyl group is transferred from acetyllipoamide to CoA to form acetyl CoA.
preserves the energy-rich thioester bond
catalyzed by dihydrolipoyl transacetylase (E2)
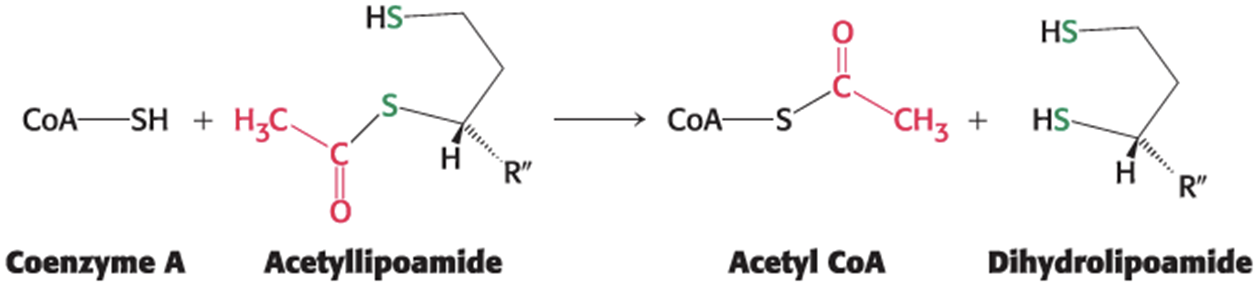
The Regeneration of Oxidized Lipoamide Step
flavoproteins = proteins tightly associated with FAD or flavin mononucleotide (FMN)
good at redox reactions
Dihydrolipoamide must be oxidized to lipoamide to regenerate the active enzyme.
two electrons are transferred to an FAD prosthetic group of the enzyme and then to NAD+
catalyzed by dihydrolipoyl dehydrogenase (E3)
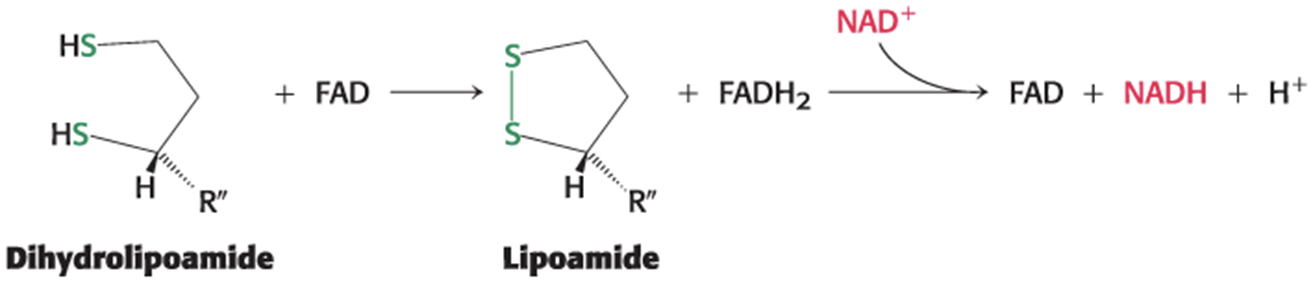
The Structure and Function of Lipoamide
The lipoamide arm of the E2 subunit carries substrates from active site to active site.
increases the overall reaction rate
minimizes side reactions
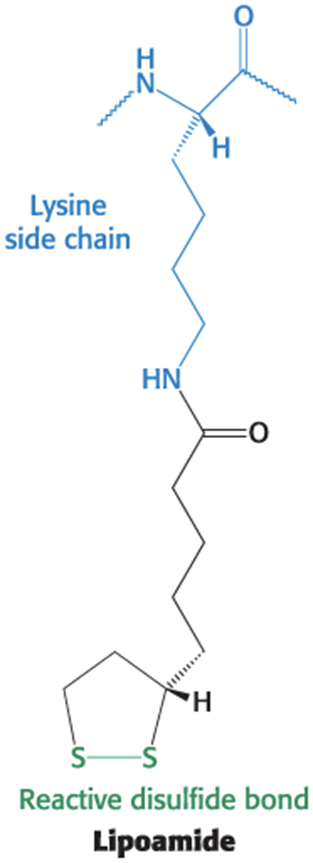
Flexible Linkages Allow Lipoamide to Move Between Different Active Sites
The core of the pyruvate dehydrogenase complex is formed by 60 molecules of E2, the transacetylase.
Transacetylase consists of 20 catalytic trimers assembled to form a hollow cube.
each trimer has three major domains:
lipoamide domain = small domain containing a bound flexible lipoamide cofactor attached to a Lys
domain interacting with E3
transacetylase domain
The Structure of the Pyruvate Dehydrogenase Complex from Bacteria Reveals a Massive Protein Complex
The core (60 molecules of E2) is surrounded in a shell of ~45 copies of E1 and ~10 copies of E3.
The Transacetylase (E2) Core Is Made Up of Three Distinct Domains
The Pyruvate Dehydrogenase Complex in Mammals
E1 is an α2β2 tetramer.
E3 is an αβ dimer.
E3-binding protein (E3-BP) = another core protein which facilitates the interaction between E2 and E3
Complex has reduced activity when missing.
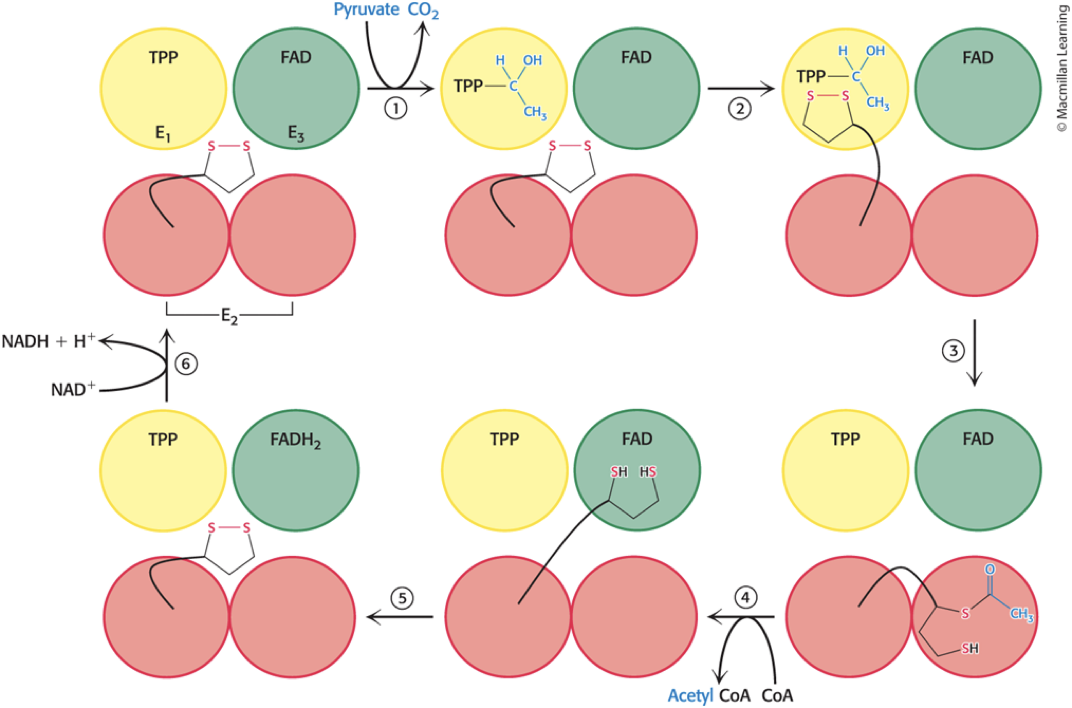
Steps in the Pyruvate Dehydrogenase Mechanism
Step 1: Pyruvate is decarboxylated at the active site of E1, forming hydroxyethyl-TPP and releasing CO2.
Step 2: E2 inserts the lipoamide arm of the lipoamide domain into the channel in E1 leading to the active site.
Step 3: E1 catalyzes the transfer of the acetyl group to the lipoamide and the acetylated arm leaves E1 and enters the E2 cube to access the E2 active site.
Step 4: The acetyl moiety is transferred to CoA, acetylCoA leaves the cube, and the reduced lipoamide arm swings to the active site of the E3 flavoprotein.
Step 5: The lipoamide is oxidized by coenzyme FAD, reactivating the lipoamide.
Step 6: NADH is produced with the reoxidation of FADH2to FAD.
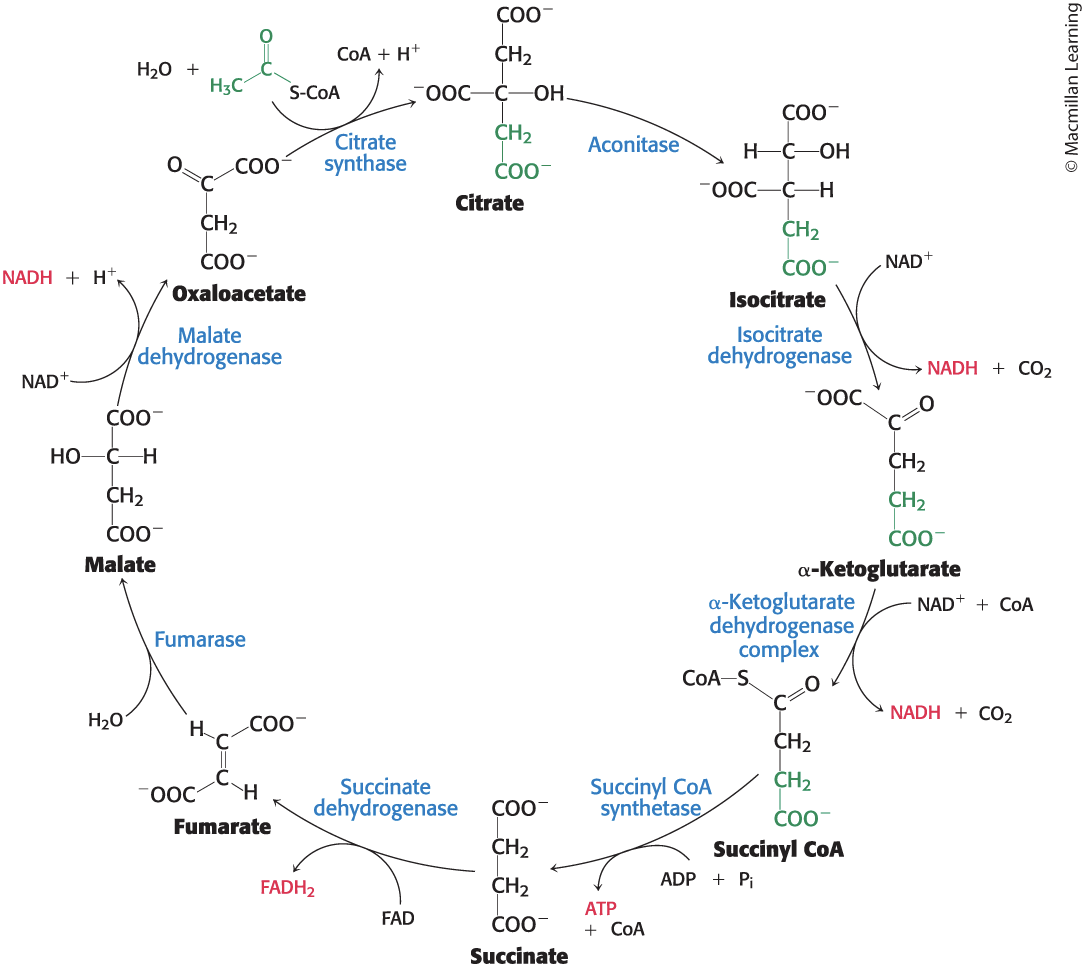
Section 17.3 The Citric Acid Cycle Oxidizes Two-Carbon Units
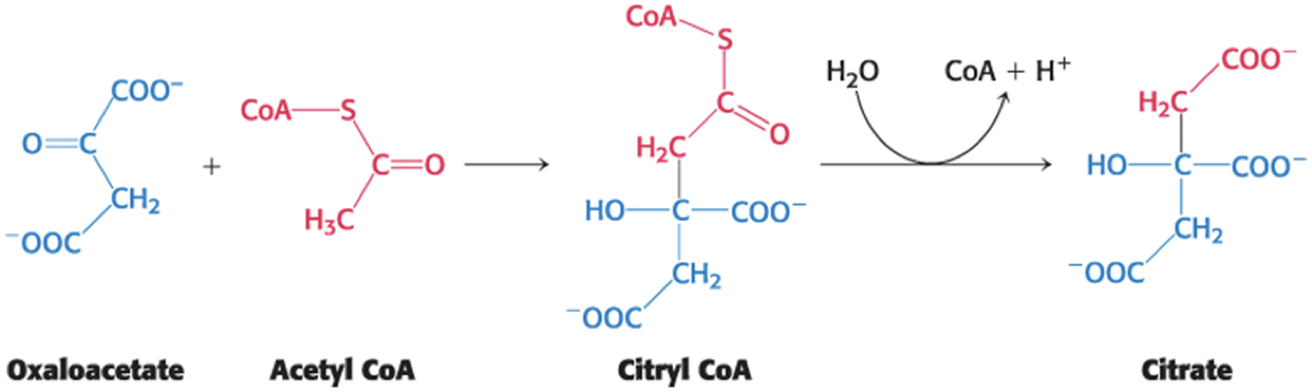
Citrate synthase catalyzes the addition of acetyl CoA and oxaloacetate, yielding citrate and CoA.
reaction is an aldol addition and a hydrolysis
proceeds through energy-rich citryl CoA
Mechanism: The Mechanism of Citrate Synthase Prevents Undesirable Reactions
It minimizes the hydrolysis of acetyl CoA to acetate and CoA side reaction
Mammalian citrate synthase is a dimer of identical subunits, each containing a small and large domain.
Active sites are in a cleft between the domains of a subunit.
Citrate synthase exhibits sequential, ordered kinetics.
Oxaloacetate binds first, followed by acetyl CoA.
Oxaloacetate induces a structural rearrangement that creates an acetyl CoA-binding site
The Ordered Binding of Substrates by Citrate Synthase Is Explained by Conformational Changes Upon Binding Oxaloacetate
Steps in the Citrate SynthaseMechanism
Step 1: His 274 donates a proton to acetyl CoA to promote the removal of a methyl proton by Asp 375 to form the enol intermediate.
enolate (?)
Step 2: Oxaloacetate is activated by the transfer of a proton from His 320 to its carbonyl carbon atom.
Step 3: Acetyl CoA attacks oxaloacetate to form a carbon–carbon double bond, His 274 is re-protonated, and citryl CoA is formed.
Step 4: His 274 participates as a proton donor to hydrolyze the thioester, yielding citrate and CoA.
The First Part of the Citrate Synthase
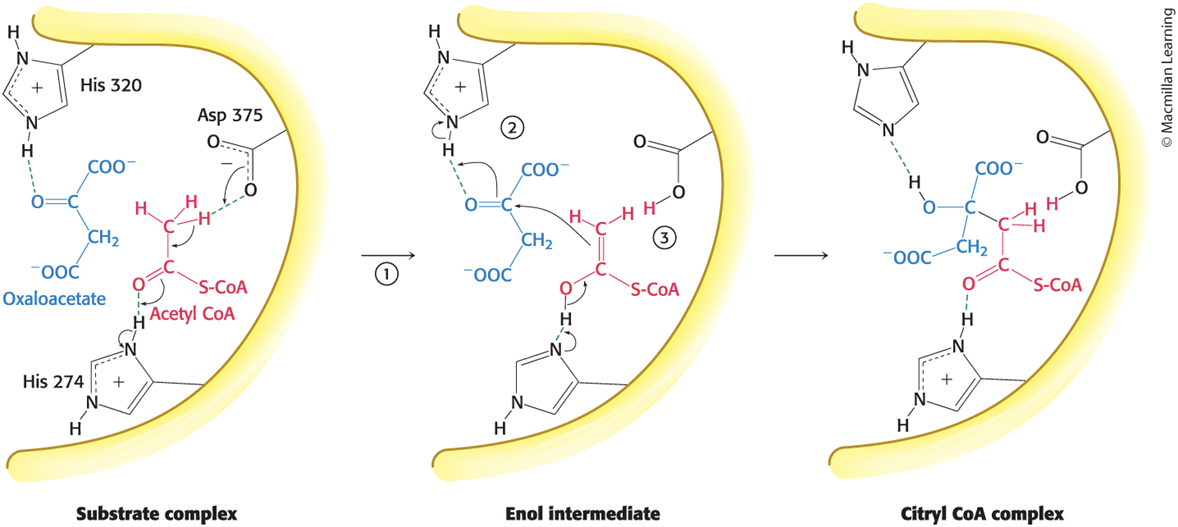
Mechanism Forms Citryl CoACitrate Is Isomerized into Isocitrate
iron-sulfur protein (nonheme iron protein) = protein that contains iron that is not bonded to heme
example: aconitase
Aconitase catalyzes the isomerization of citrate into isocitrate through a dehydration step and a hydration step.
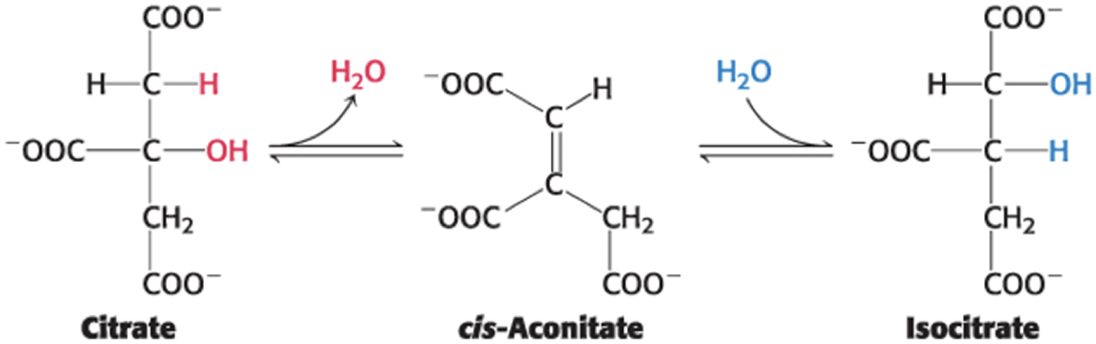
Citrate Binds Directly to the Iron–Sulfur Complex of Aconitase
Four iron atoms are complexed to four inorganic sulfides and three cysteine sulfur atoms.
One iron atom is available to bind citrate.
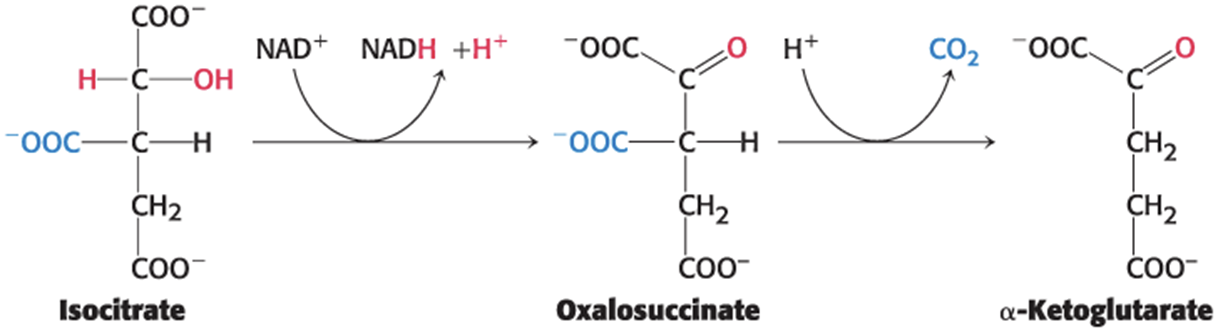
Isocitrate Is Oxidized and Decarboxylated to Alpha-Ketoglutarate
Isocitrate dehydrogenase catalyzes the oxidative decarboxylation of isocitrate, forming α-ketoglutarate and the high transfer-potential electron carrier NADH.
proceeds through the unstable oxalosuccinate
CO2 is released from oxalosuccinate to yield α-ketoglutarate
oxidative dehydrogenation
Succinyl Coenzyme A Is Formed by the Oxidative Decarboxylation of Alpha-Ketoglutarate
• The α-ketoglutarate dehydrogenase complex catalyzes the oxidative decarboxylation of α-ketoglutarate to succinyl CoA, yielding NADH.
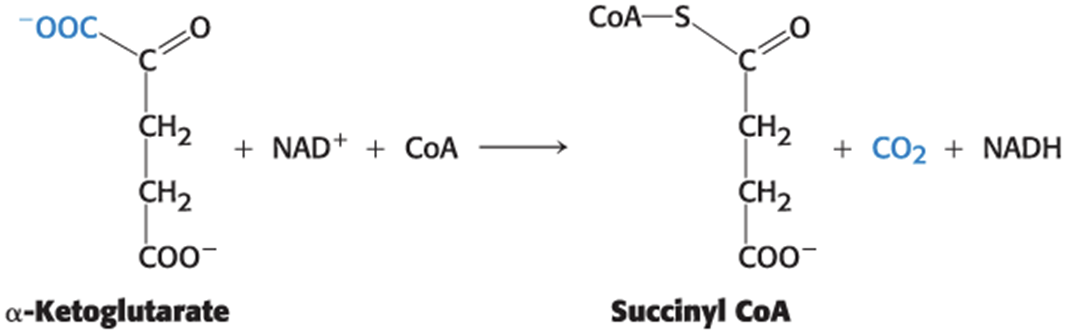
The α-Ketoglutarate Dehydrogenase Complex Is Homologous to the Pyruvate Dehydrogenase Complex
The E3 component is identical in both enzymes.
Both α-ketoglutarate and pyruvate are α-ketoacids.
Both reactions include the decarboxylation of an α- ketoacid and the formation of a thioester linkage with CoA that has a high transfer potential.
The reaction mechanisms are entirely analogous.
A Compound with High Phosphoryl-Transfer Potential Is Generated from Succinyl Coenzyme A
Succinyl CoA synthetase catalyzes the cleavage of a thioester linkage of succinyl CoA, yielding succinate.
coupled to the phosphorylation of ADP or GDP because the ∆G°′ for the hydrolysis is comparable to that of ATP
The reaction is readily reversible.
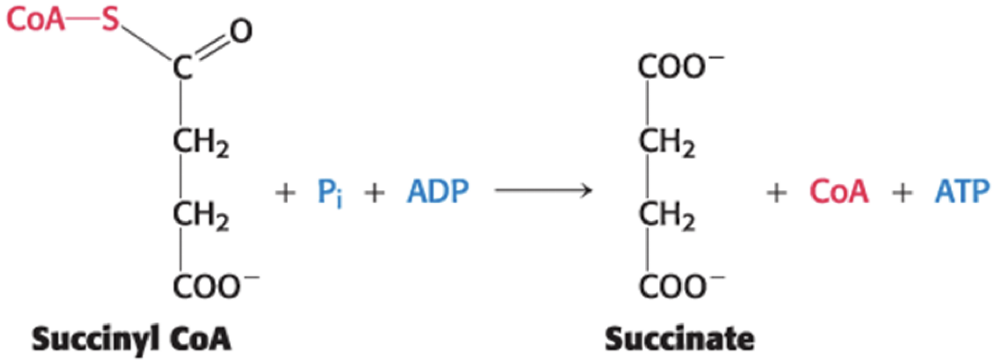
ATP or GTP Formation May Be Coupled to the Formation of Succinate
Mammals have two isozymic forms of the enzyme:
The GDP-requiring enzyme predominates in tissues performing anabolic reactions (e.g., liver), and the GTP is used to power succinyl CoA synthesis,
The ADP-requiring enzyme predominates in tissues performing large amounts of cellular respiration (e.g., skeletal and heart muscle).
Nucleoside Diphosphokinase
nucleoside diphosphokinase = catalyzes the transfer of the γ phosphoryl group from any nucleotide triphosphate to any other nucleotide diphosphate
allows for the adjustment of concentrations to meet the cell's needs
keeps concentrations in the cell near equilibrium with one another

Mechanism: Succinyl Coenzyme A Synthetase Transforms Types of Biochemical Energy
Clear example of an energy transformation: Energy inherent in the thioester molecule is transformed into phosphoryl-group-transfer potential.
The reaction is readily reversible with a ∆G°′ of −3.4 kJ mol−1
The formation of ATP at the expense of succinyl CoA is an example of substrate-level phosphorylation.
The Mechanism of Succinyl CoA Synthetase Allows the Formation of a Phosphoanhydride Through a Phosphorylated Enzyme Intermediate
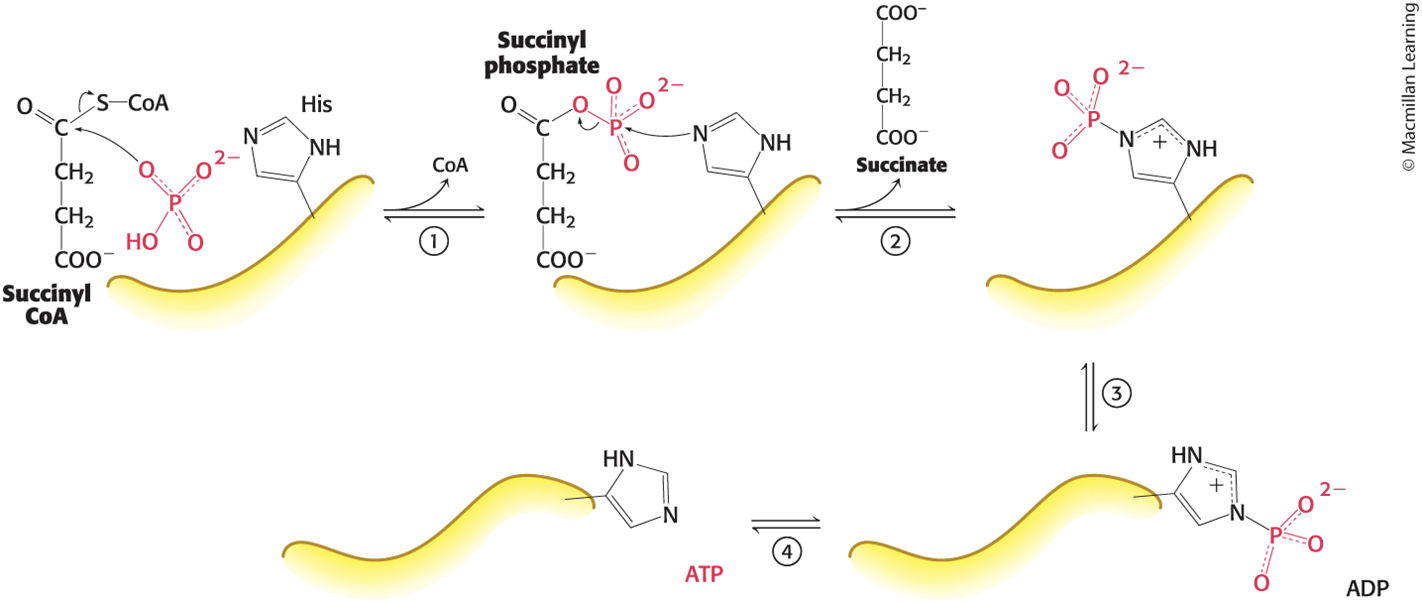
addition elimination, not SN2
SN2 like reaction
Steps in the Succinyl Coenzyme A Synthetase Mechanism
Step 1: Orthophosphate attacks succinyl CoA, displacing coenzyme A and generating succinyl phosphate, an energy-rich compound.
Step 2: A His residue acts as a moving arm that removes the phosphoryl group, forming phosphohistidine and releasing succinate.
Step 3: The phosphohistidine swings over to a bound ADP.
Step 4: The phosphohistidine transfers the group to ADP to form ATP.
Oxaloacetate Is Regenerated by the Oxidation of Succinate
Succinate dehydrogenase, fumarase, and malate dehydrogenase catalyze successive reactions of four-carbon compounds to regenerate oxaloacetate.
FADH2 and NADH are generated.
Once regenerated, oxaloacetate can initiate another cycle.
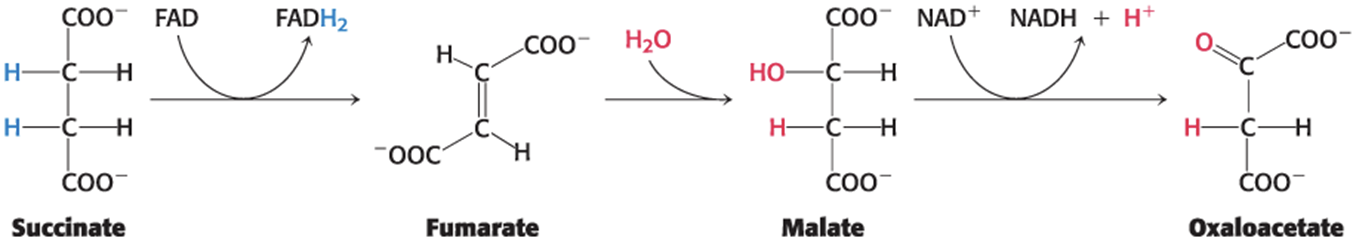
Succinate Is Oxidized to Fumarate by Succinate Dehydrogenase
Succinate dehydrogenase:
is an iron–sulfur protein.
has the isoalloxazine ring of FAD covalently attached to a histidine side chain.
is embedded in the inner mitochondrial membrane.
is directly associated with the electron-transport chain.
FAD is the hydrogen acceptor because the free-energy change is insufficient to reduce NAD+

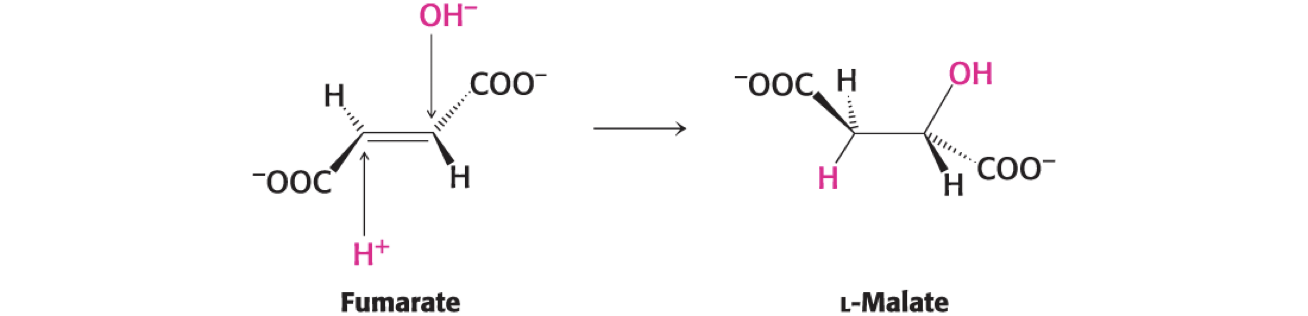
.Fumarate Is Hydrated to L-Malate by Fumarase
Fumarase catalyzes the stereospecific trans addition of H+ and OH−, yielding only the L-isomer of malate.
one specific stereoisomer
Malate Is Oxidized to Oxaloacetate by Malate Dehydrogenase
Malate dehydrogenase catalyzes the oxidation of malate, yielding oxaloacetate and NADH.
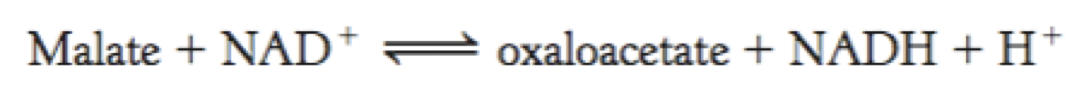
∆G°′ is significantly positive (∆G°′= +29.7 kJ mol−1).
The reaction is driven by the use of the products: oxaloacetate by citrate synthase and NADH by the electron-transport chain.
the fact that we use all the products helps move the reaction forward
good spot for regulation
The Citric Acid Cycle Produces High Transfer-Potential Electrons, ATP, and CO2
The net reaction of the citric acid cycle is
Acetyl CoA + 3 NAD+ + FAD + ADP + Pi + H2O → 2 CO2 + 3 NADH + FADH2 + ATP + 2H+ + CoA
The two carbon atoms that enter each cycle as acetyl CoA are not the ones that leave as CO2 during the initial two decarboxylation reactions.
The Stoichiometry of the Citric Acid Cycle
Two carbon atoms enter in the form of acetyl CoA, and two carbons leave in the form of CO2 molecules.
Four pairs of hydrogen atoms leave in four oxidation reactions (yielding three NADH and one FADH2).
One compound with high phosphoryl-transfer potential (usually ATP) is generated.
Two water molecules are consumed.
One Acetyl Unit Generates Approximately 10 Molecules of ATP
When oxidized via the electron-transport chain:
each pair of electrons from NADH will generate ~2.5 ATP.
each pair of electrons from FADH2 will generate ~1.5 ATP.
Nine high transfer-potential phosphoryl groups aregenerated from the oxidation of 3 NADH and 1 FADH2 molecules.
One ATP is directly formed in one round of the citric acid cycle.
Eight Enzyme-Catalyzed Reactions Make Up the Full Citric Acid Cycle
There is a physical association of the citric acid cycle enzymes into a supramolecular complex.
allows for substrate channeling
Section 17.3 Entry to the Citric Acid Cycle and Metabolism Through It Are Controlled
The formation of acetyl CoA from pyruvate is irreversible in animal cells.
Acetyl CoA has two principal fates:
metabolism by the citric acid cycle
incorporation into lipids
The activity of the pyruvate dehydrogenase complex is tightly controlled allosterically and by reversible phosphorylation.
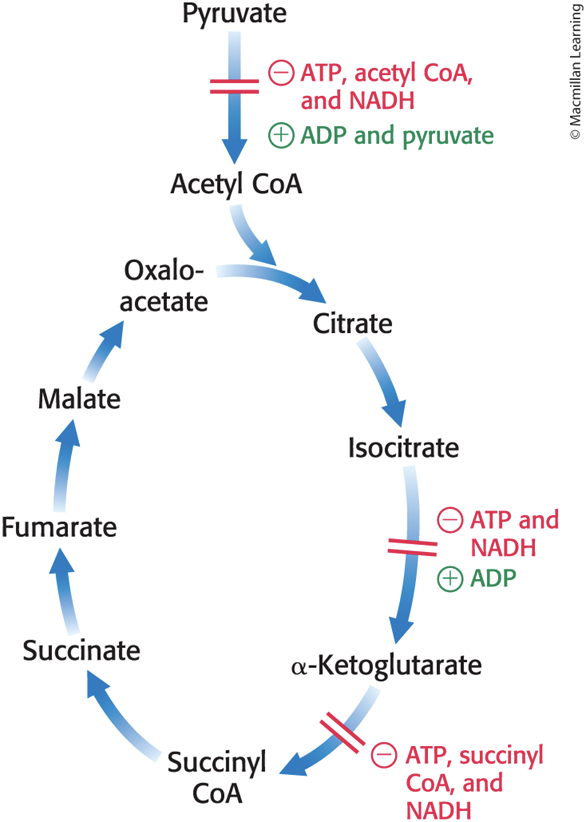
The Pyruvate Dehydrogenase Complex Is Regulated Allosterically
High concentrations of reaction products inhibit the reaction by informing the enzyme that there is no need to metabolize pyruvate to acetyl CoA:
Acetyl CoA inhibits the transacetylase component (E2).
NADH inhibits the dihydrolipoyl dehydrogenase (E3).
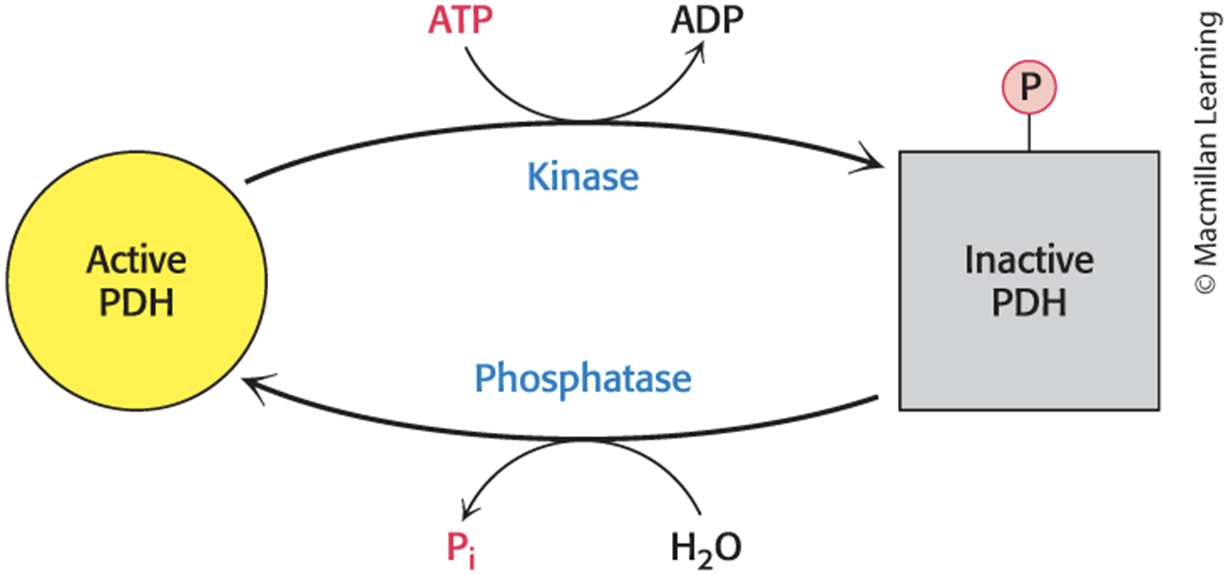
The Pyruvate Dehydrogenase Complex Is Regulated by Reversible Phosphorylation
Pyruvate dehydrogenase kinase (PDK) phosphorylates the pyruvate dehydrogenase component (E1).
switches off the activity of the complex
Pyruvate dehydrogenase phosphatase (PDP) dephosphorylates E1.
phosphatases hydrolyze with water
In mammals, both PDK and PDP are associated with the E2-E3-BP core complex.
The Activity of the Pyruvate Dehydrogenase Complex Is Regulated by Reversible Phosphorylation
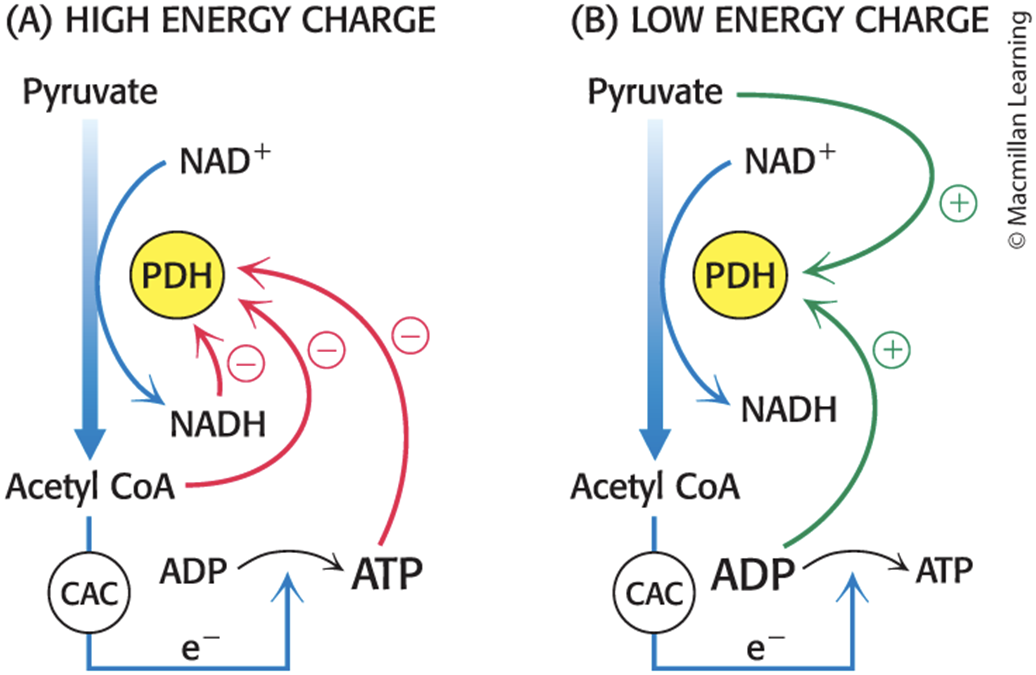
The Pyruvate Dehydrogenase Complex Responds to Changes in the Energy Charge of the Cell
Regulation in Biological Conditions
At rest, the ratios of NADH/NAD+, acetyl CoA/CoA, and ATP/ADP are high.
promotes phosphorylation and inactivation of the complex by activating PDK
During activity:
high ADP and pyruvate activate the complex by inhibiting the kinase.
Ca2+ stimulates the phosphatase, enhancing pyruvate dehydrogenase activity
In Some Tissues, the Phosphatase Is Regulated by Hormones
In liver tissue, epinephrine binds to the α-adrenergic receptor, causing an increase in Ca2+ concentration that activates the phosphatase.
In liver and adipose tissue, insulin stimulates the phosphatase.
Pyruvate Dehydrogenase Phosphatase Deficiency
Individuals with pyruvate dehydrogenase phosphatase deficiency have a pyruvate dehydrogenase complex that is always phosphorylated (i.e., inactive).
In these individuals, glucose is processed to lactate rather than to acetyl CoA.
resulting in lactic acidosis
many tissues malfunction in the acidified environment, including the central nervous system
Diabetic Neuropathy May Be Due to Inhibition of the Pyruvate Dehydrogenase Complex
diabetic neuropathy = numbness, tingling, or pain in the hands, arm, fingers, toes, feet, and legs
common complication (~50% of patients) of both type 1 and type 2 diabetes
can be treated with painkillers, but cannot be cured
overproduction of lactic acid by cells in the dorsal root ganglion may be a significant contributor
hyperglycemia (high glucose concentration) = the defining feature of diabetes
increases PDK activity in the cells of the dorsal root ganglion, leading to inhibition of the pyruvate dehydrogenase complex
Inhibition of the complex leads to pyruvate being processed to lactate.
Excess lactate leads to an increase in acid-sensing nociceptors (pain receptors), a type of G-protein-coupled receptor, resulting in diabetic neuropathy.
The Citric Acid Cycle Is Regulated at Several Points
Isocitrate dehydrogenase and α-ketoglutarate dehydrogenase are allosteric enzymes that primarily regulate the rate of cycling.
These are the first two enzymes that harvest high-energy electrons in the cycle.
Section 17.5 The Citric Acid Cycle Is a Source of Biosynthetic Precursors
• The citric acid cycle integrates many of the cell's other metabolic pathways, including those of carbohydrates, fats, amino acids, and porphyrins.
• Many citric acid cycle components are precursors for biosynthesis of key biomolecules.
The Citric Acid Cycle Plays an Important Role in Biosynthesis
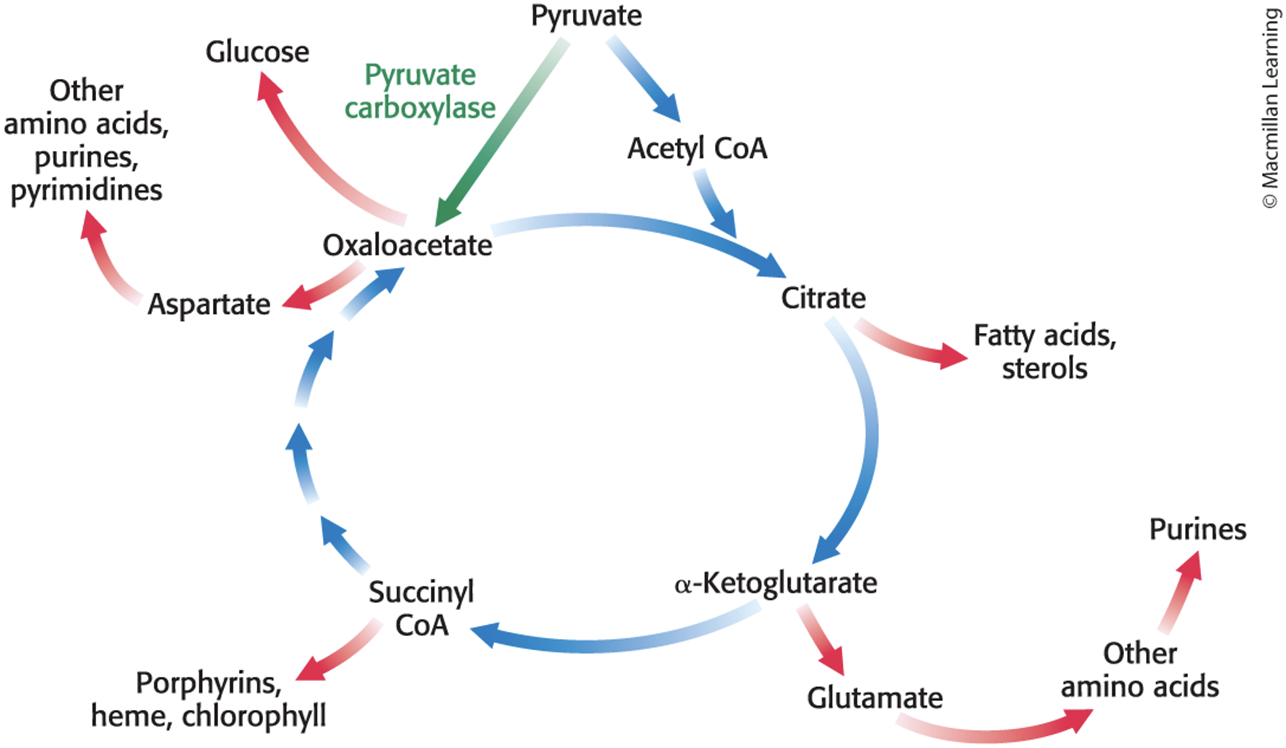
The Citric Acid Cycle Must Be Capable of Being Rapidly Replenished
Citric acid cycle intermediates must be replenished if any are used for biosyntheses.
Mammals lack the enzymes for the net conversion of acetyl CoA into oxaloacetate or other cycle intermediate.
anaplerotic reaction = a reaction that leads to the net synthesis, or replenishment, of pathway components
Pyruvate carboxylase catalyzes the formation carboxylation of pyruvate to oxaloacetate.
This reaction is used in gluconeogenesis and is dependent on the presence of acetyl CoA.
The Disruption of Pyruvate Metabolism Is the Cause of Beriberi and Poisoning by Mercury and Arsenic
beriberi = a neurologic and cardiovascular disorder is caused by a dietary deficiency of thiamine (vitamin B1)
Thiamine is the precursor of the cofactor thiamine pyrophosphate (TPP).
TPP is the prosthetic group of:
pyruvate dehydrogenase.
α-ketoglutarate dehydrogenase.
transketolase (a pentose phosphate pathway enzyme).
Patients with beriberi have higher than normal levels of pyruvate and α-ketoglutarate in the blood.
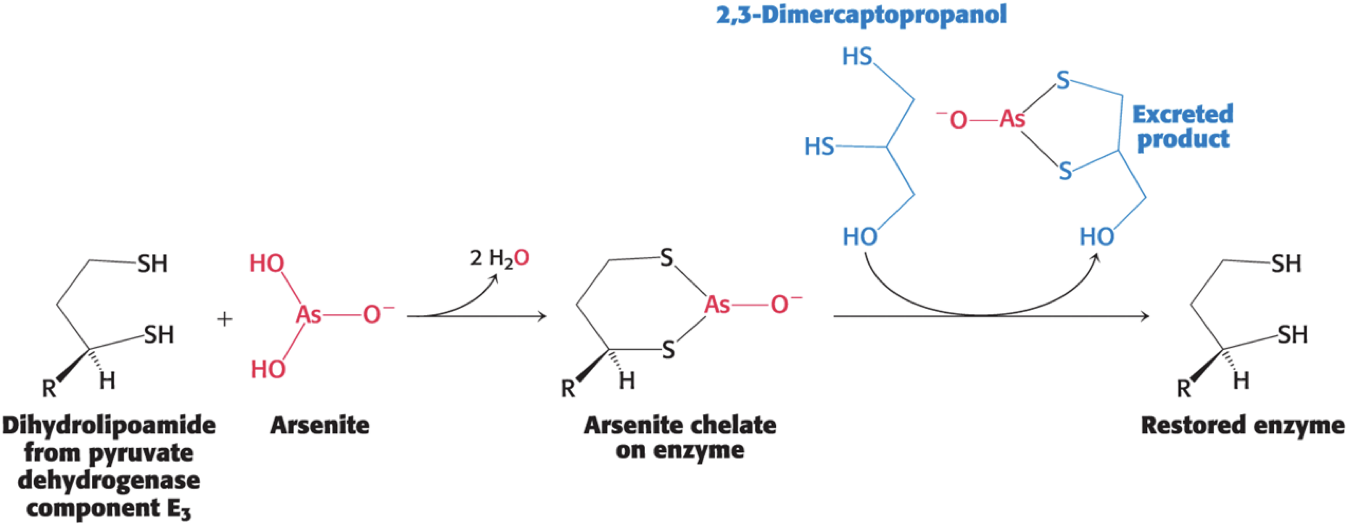
Mercury and Arsenite Inhibit the Pyruvate Dehydrogenase Complex
Both mercury and arsenite (AsO33−) have a high affinity for neighboring sulfhydryls.
Binding of mercury or arsenite to the dihydrolipoyl groups of the E3 component of the pyruvate dehydrogenase complex inhibits the complex and leads to central nervous system pathologies.
Treatment is the administration of sulfhydryl reagents with adjacent sulfhydryl groups to compete with the dihydrolipoyl residues for binding.
Arsenite Inhibits the Pyruvate Dehydrogenase Complex