1.6 Trends In Periodic Table
Trends in First Ionisation Energy
Across a Period (Left to Right)
General Trend:
First ionisation energy increases across a period.
Explanation:
Nuclear Charge Increases:
As you move across the period, the number of protons in the nucleus increases, leading to a stronger attraction between the nucleus and the outermost electrons.Shielding Remains Constant:
Electrons are added to the same principal energy level (same shell), so shielding from inner electrons does not change significantly.Atomic Radius Decreases:
The increased nuclear attraction pulls the outer electrons closer to the nucleus, reducing the atomic radius.Stronger Nuclear Attraction:
Due to the higher nuclear charge and reduced distance from the nucleus, more energy is required to remove the outer electron.
Anomalies:
Group 2 to Group 3 (e.g., Be to B):
The ionisation energy decreases slightly because the outer electron in Group 3 is in a higher-energy ppp-orbital, which is further from the nucleus and shielded by sss-electrons.Group 5 to Group 6 (e.g., N to O):
The ionisation energy decreases slightly because Group 16 elements have paired electrons in a ppp-orbital. The repulsion between paired electrons makes it easier to remove one.
Down a Group (Top to Bottom)
General Trend:
First ionisation energy decreases down a group.
Explanation:
Atomic Radius Increases:
As you move down the group, additional electron shells are added, increasing the distance between the nucleus and the outermost electron.Increased Shielding:
More inner electron shells shield the outermost electron from the attractive force of the nucleus.Weaker Nuclear Attraction:
Although nuclear charge increases, the effects of increased distance and shielding outweigh the increased nuclear charge, making it easier to remove the outermost electron.
Key Trends to Remember
Across a Period: First ionisation energy increases due to stronger nuclear attraction.
Down a Group: First ionisation energy decreases due to increased shielding and atomic radius.
Anomalies: Look for deviations caused by sub-shell structure and electron pairing.
Summary:
Across a period: First ionisation energy increases due to increasing nuclear charge and decreasing atomic radius.
Down a group: First ionisation energy decreases due to increased shielding and atomic radius.
Changes in sub-shell configuration and electron pairing cause anomalies in trends.
/
Trends in melting point
Down group II
All of these elements are held together by metallic bonds. The melting points generally get lower as you go down the group because the metallic bonds get weaker.
The atoms in a metal are held together by electrostatic forces. As the atoms get more significant, the nuclei get further away from the delocalised electrons, meaning they can change states more easily.
Oxidation numbers
Rules:
Uncombined elements have an oxidation number of 0 (e.g., O₂ = 0).
The sum of oxidation numbers in a compound is 0 (e.g., MgO: Mg = +2, O = -2, total = 0).
In an ion, the sum equals its charge (e.g., NO₃⁻: N = +5, O = -2, total = -1).
Group 1 metals = +1, Group 2 metals = +2.
Group 6 elements = typically -2, Group 7 elements = typically -1.
Oxygen is -2, except in peroxides (-1) or with fluorine.
Hydrogen is +1, except in metal hydrides (-1) (e.g., NaH: Na = +1, H = -1).
S-block elements react with oxygen
S-block metals form oxides when exposed to oxygen or air.
Group 1 metals develop a black oxide coating quickly and are stored under oil to prevent this.
Reaction equations:
Group 1: 4M+O2→2M2O4M + O_2 → 2M_2O4M+O2→2M2O (e.g., Li: 4Li+O2→2Li2O4Li + O_2 → 2Li_2O4Li+O2→2Li2O)
Group 2: 2M+O2→2MO2M + O_2 → 2MO2M+O2→2MO (e.g., Sr: 2Sr+O2→2SrO2Sr + O_2 → 2SrO2Sr+O2→2SrO)
Oxides are basic and react with acids to form salt and water (e.g., CaO+2HCl→CaCl2+H2OCaO + 2HCl → CaCl_2 + H_2OCaO+2HCl→CaCl2+H2O).
S-block elements react with water
Group 1 & 2 metals react with water to form hydroxides and release hydrogen, causing effervescence.
Group 1 metals become more reactive down the group; from potassium onwards, hydrogen may ignite.
Reaction: 2M+2H2O→2MOH+H22M + 2H_2O → 2MOH + H_22M+2H2O→2MOH+H2 (e.g., Li: 2Li+2H2O→2LiOH+H22Li + 2H_2O → 2LiOH + H_22Li+2H2O→2LiOH+H2)
Group 2 reactions vary:
Beryllium does not react.
Magnesium reacts slowly with water but reacts with steam to form oxide:
Mg+H2O→MgO+H2Mg + H_2O → MgO + H_2Mg+H2O→MgO+H2
Other Group 2 metals (Ca, Sr, Ba) follow:
M+2H2O→M(OH)2+H2M + 2H_2O → M(OH)_2 + H_2M+2H2O→M(OH)2+H2
(e.g., Ca: Ca+2H2O→Ca(OH)2+H2Ca + 2H_2O → Ca(OH)_2 + H_2Ca+2H2O→Ca(OH)2+H2)
The reactions of aqueous Mg2+, Ca2+ and Ba2+ with OH-, CO32- and SO42- ions
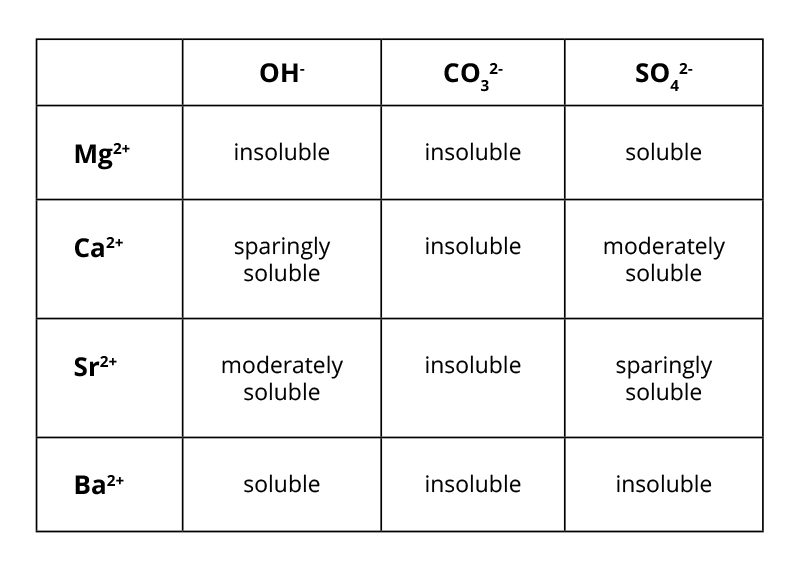
All precipitates are white.
You need to know ionic equations from this table.
For example:
Mg2+(aq)+CO32−(aq) → MgCO3(s)
General trends in reactivity of s-block elements
Reactivity increases down both Group 1 and Group 2 because these elements need to lose their outer electrons to react.
Atomic size increases down the group, meaning outer electrons are further from the nucleus and experience a weaker electrostatic attraction, making them easier to lose.
Shielding effect increases as extra energy levels are added, reducing the nucleus’s pull on outer electrons. This lowers ionization energy, making it easier to form positive ions (cations).
This trend is clearly observed in reactions with water and air, which become more vigorous down the group.
Group 1 metals are always more reactive than Group 2 because they only need to lose one electron, whereas Group 2 metals need to lose two electrons, requiring more energy.
Base strength of Group 1 and Group 2 oxides and hydroxides
All Group 1 & Group 2 oxides and hydroxides are basic, reacting with acids to form salt and water
Group 1 bases are stronger than Group 2 bases, and base strength increases down the group.
Base strength depends on how readily the base dissociates into ions in solution (e.g., NaOH completely dissociates:
Uses of bases depend on strength:
Strong bases (e.g., NaOH) are used in soaps and industrial cleaners.
Weaker bases (e.g., Mg(OH)₂) are used in antacids to neutralize stomach acid.
Ca(OH)₂, a moderate-strength base, is used in farming to neutralize soil.
Thermal Decomposition:
Group 2 hydroxides decompose on heating to the oxide and steam.
e.g. Ca(OH)2(s) CaO(s) + H2O(g)
All Group 2 carbonates decompose on heating to the oxide and carbon dioxide.
e.g. MgCO3(s) MgO(s) + CO2(g)
Thermal stability increases in both as you go down the group.
The trend for carbonates can be shown in the laboratory by heating them and seeing how long it takes the CO2 formed to turn limewater cloudy.