Yr9-C2: The periodic table:
C2.1. Development of the periodic table
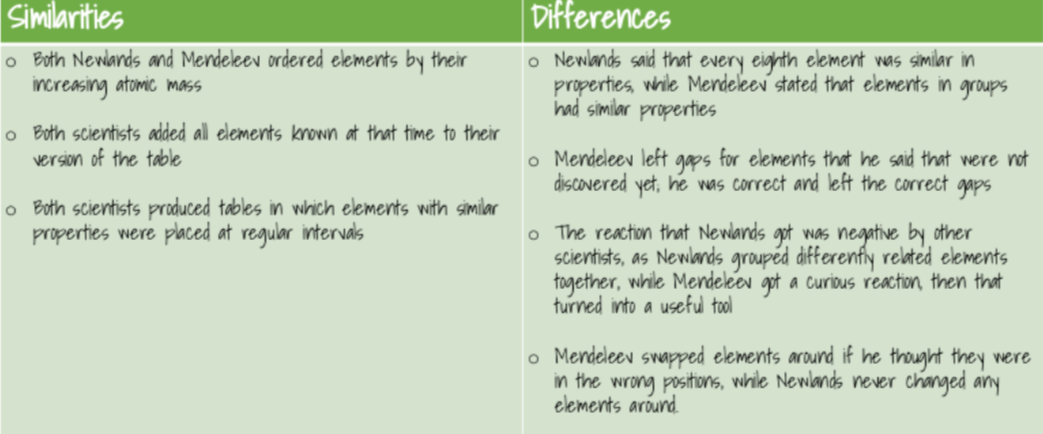
Before the discovery of protons, neutrons and electrons, scientists attempted to classify the elements by arranging them by atomic mass. John Newlands decided this. (Now is arranged by atomic number)
John Newlands:
English Analytical Chemist in the 1800s.
He arranged the elements on the periodic table by their
atomic mass
He called this arrangement the Law of
Octaves
He found a pattern.The pattern showed that each element was similar to the element eight places ahead of it.
Newlands noticed that the properties of every eighth element seemed similar.
When he was filling in his octaves table he didn’t consider if there were any more elements, undiscovered.
By ordering strictly according to atomic mass, Newlands was forced to put some elements into groups which did not match their chemical properties.
Eg - he put iron in the same group as oxygen and sulphur (metal and two non-metals)
The early periodic tables were incomplete and some elements were placed in inappropriate groups if the strict order of atomic mass was followed.
So someone new came along with a brand new idea…
Dmitri Mendeleev:
Russian Chemist in 1800s.
In 1869, Mendeleev also arranged the elements known at the time
in order of atomic mass
He realised that the physical and chemical properties of elements were related to their atomic mass in a 'periodic' way, and arranged them so that groups of elements with similar properties fell into vertical columns in his table.
Mendeleev left spaces in his periodic table for undiscovered elements.
Mendeleev predicted the atomic mass and chemical/physical properties of the missing elements.e.g Gallium
C2.2 Electronic structures and the periodic table
Electrons fill the shells closest to the nucleus unless there is no space left in those shells
Shell 1 holds a maximum of 2 e–
Shell 2 holds a maximum of 8 e–
Shell 3 holds the next 8 e–
Shell 4 holds the next 2 e–
This works for the first 20 elements
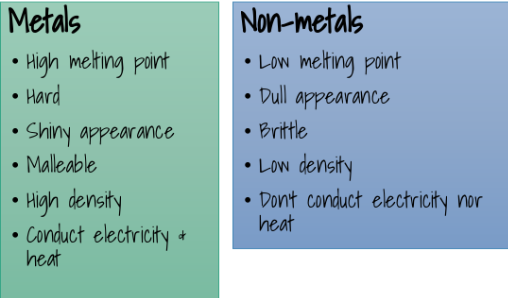
Non Metals and metals:
 Group 1: Alkali Metals:
Group 1: Alkali Metals:
have 1 electron in outer shell
soft metals (can cut with knife)
low melting points
low density (some float on water)
very reactive (as easy to lose one electron)
react with water to give H2 and an alkaline solution
Group 7: Alkali Metals:
have 7 electrons in outer shell
non-metals
toxic
coloured vapours
exist as diatomic molecules, e.g. Cl2
very reactive (as easy to gain one electron)
Group 0:
stable electron structure (‘full outer shells’)
non-metals
colourless gases
NOT REACTIVE (as they already have stable electron structure)
C2.3 Alkali Metals:
The elements in group 1, on the left of the periodic table, are called the alkali metals.
These metals are all very reactive and are rarely found in nature as
pure elements.
The alkali metals are so reactive that, as elements, they have to be stored in oil. This stops them reacting with oxygen in the air.
The elements in group 1 also react with water and form alkaline compounds. This is why they are called alkali metals.
What are the properties?
They are soft and can be cut by a knife.
Softness increases going down the group.
They have a low density.
Lithium, sodium and potassium float on water.
They have low melting and boiling points.
Sodium and potassium melted into a sphere.
The alkali metals generally become more dense going down the group, except for potassium.
The melting and boiling points decrease going down group 1 because the atoms get larger. Therefore the attraction between the nucleus and the delocalised electrons is weaker.
Reactivity:
Reactivity increases down the group.
Reactions all involve the loss of the outermost electron.
Losing this electron seems to get easier as we go down the group.Because the bigger the atom,the further the outer electron is away from the nucleus and so the easier it is to lose. (the electrostatic force of attraction between the protons in the nucleus and the electrons get weaker)
Reaction with water:
alkali metal + water → alkali metal hydroxide + hydrogen
e.g lithium: lithium +water → lithium hydroxide + Hydrogen
Reaction with oxygen: (oxidation)
alkali metal + oxygen →alkali metal oxide
lithium + oxygen → lithium oxide
C2.4 Halogens:
The elements in group 7 are called the halogens.
Halogens react with metals and are poisonous non-metals.
They are characterised by coloured vapours.
Halogens have low melting and boiling points.
They are poor conductors of both heat and electricity.
Halogens all have 7 electrons in their outermost shell and so all need to gain just 1 more electron to get a full outer shell. Therefore, halogens are highly reactive, tending to gain an electron in chemical reactions to achieve a full outer shell.
The halogens can do this by either sharing electrons (bonding covalently) or gaining an electron (bonding ionically).
C2.5 Explaining Trends
Physical property:
Any characteristic that can be determined without changing the substance's chemical identity.
Examples include colour, density, hardness, and melting and boiling points
Chemical property:
Any characteristic that can be determined only by changing a substance's molecular structure
Examples include flammability, toxicity, acidity, reactivity (many types), and heat of combustion.
Why does the reactivity of elements in group 7 decrease as you go down the group?
As you go down the group the atom gets bigger.
This means that it becomes harder for the atom to gain one electron in its outermost shell.
This is because the outer shell is further away from the nucleus and so electrons are less strongly attracted to it.
Further down group 7 the electron becomes harder to gain and so elements as you go down the group become less and less reactive.
C2.6 The transition elements:
Transition metals are a collection of elements in the centre of the periodic table – between the group 2 and 3.
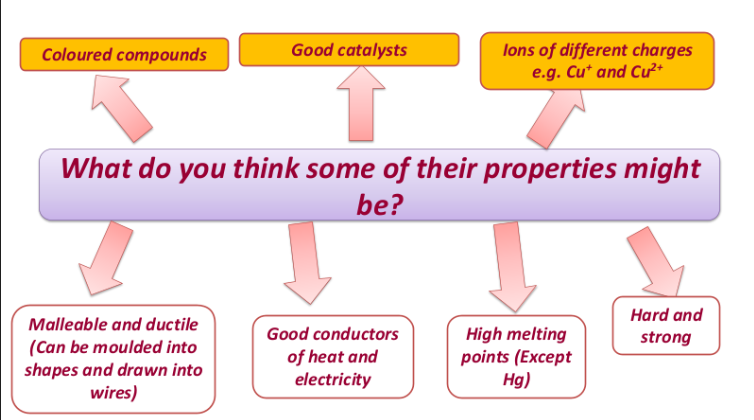 Transition metals can also be used as catalysts in certain reactions
Transition metals can also be used as catalysts in certain reactions
E.g.Iron is a catalyst in the Haber process
Platinum is a catalyst in production of nitric acid
Nickel in hydrogenation of vegetable oils
Transition metals don’t have the same number of electrons on their outer shells.
This means different ions (and charges) can be formed as the valencies are different