Quiz 1: Isotopes, Ions, and Average Atomic Mass 9th Grade Chem
This quiz will cover isotopes, ions, and average atomic mass. You will have 20 minutes to take it in class. It will be all fill in the blank and calculation-based questions. BRING A PENCIL AND A CALCULATOR!
Mass number: Protons+neutrons
when given the mass number, subtract the amount of protons from the mass number (see atomic number), and what you’re left with is the amount of neutrons
Atomic number: protons
The larger number on the periodic table
Atomic mass: average of the relative amounts of each isotope
Electrons: Negatively charged particles, found outside of the nucleus of an atom
Protons: positively charged particles, found in the nucleus of an atom
Neutrons: uncharged particles, found in the nucleus of an atom
Neutral atoms: have the same amount of electrons and protons; neutrons differ
Isotopes: atoms of the same element with the same amount of protons and a differing amount of neutrons
the notation for this is has in subscript:
on the top left corner before the chemical symbol, the mass number
on the bottom left corner before the chemical symbol, the atomic number
Ex:
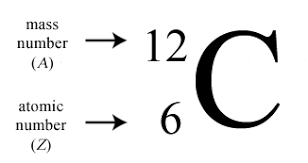
It can also be written with the element name, a dash, and then following that the mass number
Ex: Carbon-12
Ions:
there is not an equal amount of protons and electrons
if there are more protons than electrons, the charge is positive
Ex: Helium has a charge of +2. I know that it has 2 protons, but because it has a charge of +2, there are 2 more protons than electrons. Therefore, it has 0 electrons.
if there are more electrons than protons, the charge is negative
Ex: nitrogen has a charge of -3. I know that it has 7 protons, but because it has a charge of -3, there are 3 more electrons than protons. Therefore, there are 10 electrons.
Calculating average atomic mass (AAM): (atomic mass of isotope #1)(fractional abundance of isotope #1)+(atomic mass of isotope #2)(fractional abudnace of isotope #2)
fractional abundance is the percentage of a particular isotope in the total sample of atoms, written as a decimal
remember sig figs!!!
Significant figures
When multiplying/dividing sig figs, whichever # has the least amount of sig figs, you’ll round there
when adding/subtracting sig figs, line it up all the way. Whichever one is the shortest, go from there