Conservation of Energy
1.1 Law of Conservation of Energy
States the idea that energy can neither be created nor be destroyed. Although, it can change into a different forms.
The total energy of an isolated system is always constant when all forms of energy are taken into account.
All forms of energy follow the law of conservation of energy.
In brief, the law of conservation of energy states that in a closed system, i.e. a system that is isolated from its surroundings, the total energy of the system is conserved.
The first law of thermodynamics is the law of conservation of energy. The concept was first postulated and tested in the 18th century by Émilie du Châtelet, a French mathematician and philosopher.
Energy Stores
Energy is stored in objects in many different energy stores. Here are some common energy stores:
Kinetic Energy: the energy associated with the motion of an object. The faster an object moves, the more kinetic energy it possesses.
Potential Energy: the energy stored in an object based on its position or state. Gravitational potential energy depends on an object's height, and elastic potential energy is stored in objects that can be stretched or compressed.
Chemical Energy: energy stored in the bonds between atoms and molecules. This energy is released during chemical reactions.
Thermal Energy: the internal energy of a system due to the random motion of its particles. It is associated with the temperature of an object.
Nuclear Energy: energy stored in the nucleus of an atom. It can be released through nuclear reactions, such as fission or fusion.
Electrostatic Energy: energy stored in charged particles due to their positions relative to each other.
Magnetic Energy: energy stored in a magnetic field. It is associated with the alignment and motion of magnetic materials.
Energy Transfer Pathways
Energy is transferred between stores through transfer pathways.
Examples of these are: mechanically, electrically, by heating, by radiation.
Mechanical working: when a force acts on an object e.g. pulling, pushing, stretching, squashing
Electrical working: a charge (current) moving through a potential difference e.g. charge flowing around a circuit
Heating by particles: energy is transferred from a hotter object to a colder one
Heating by radiation: energy transferred by electromagnetic waves, e.g. Light
Representing Energy Transfers
Energy Flow Diagram
A flow diagram is a useful tool for representing energy stores and transfers.
This displays the stores and transfers that occur inside a system.
Sankey Diagrams
One way to depict energy transfers is with Sankey diagrams.
The splitting arrows in Sankey diagrams, which display the ratios of the energy transfers occurring, are what distinguish them.
The various energy exchanges are represented by the various segments of the arrow in a Sankey diagram:
The energy into the system is represented by the flat end on the left side of the arrow.
The useful energy production is shown by the straight line heading to the right, which indicates the energy that is ultimately stored at the desired location.
The wasted energy is symbolised by the arrows that bend away.
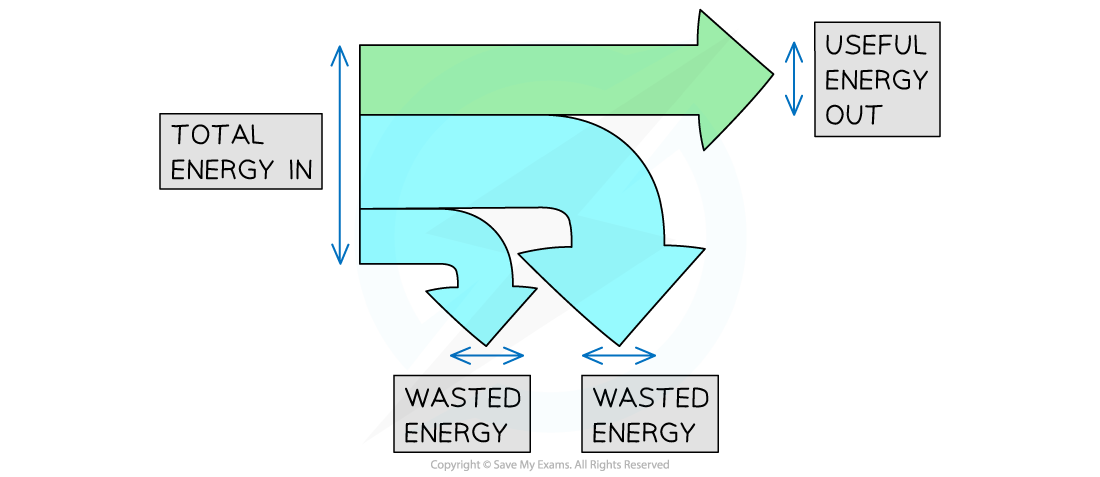
Each arrow's width corresponds to the quantity of energy being transferred.
Consequently to the energy discussion:
Energy in total = Energy used up + Energy wasted
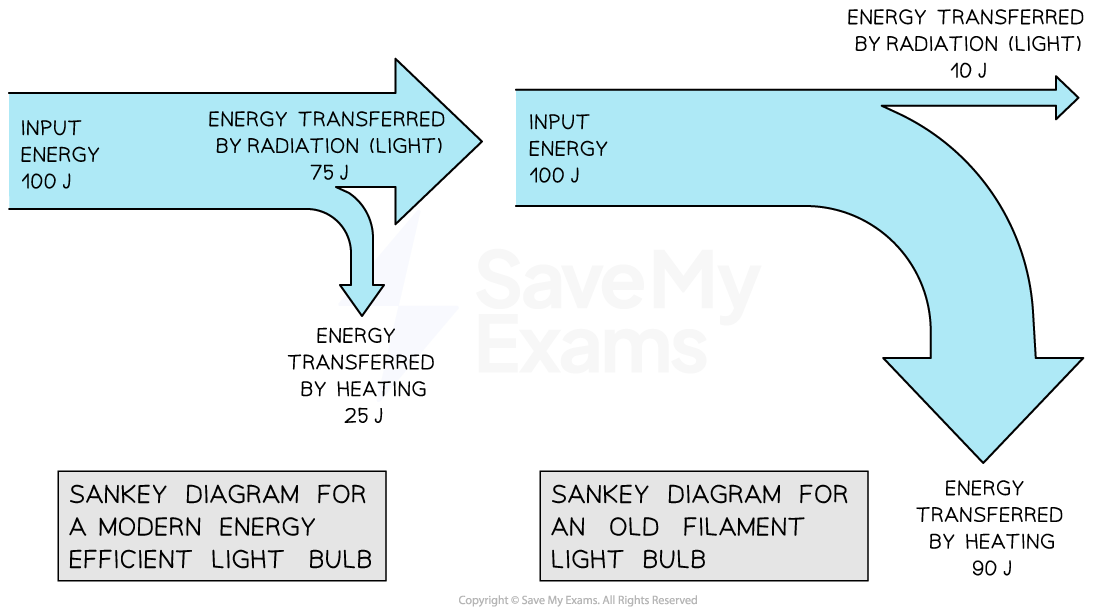
An old filament light bulb's Sankey diagram will not resemble the one for a contemporary, energy-efficient light bulb.
Less energy is lost by a lightbulb with higher efficiency.
The smaller downward-pointing arrow, which stands for heat energy, illustrates this.
1.2 Gravitational Potential Energy
The definition of energy in an object's gravitational store is the energy that an object in a gravitational field possesses because of its height.
This implies that energy is transferred to an object's gravitational potential store when it is raised.
Energy will be released from an object's gravitational potential store if it falls.
The following equation can be used to determine an object's change in gravitational potential energy, or ΔGPE:
ΔGPE = m × g × Δh
Where:
ΔGPE is the gravitational potential energy change, expressed in joules (J).
m = mass (kg) in kilogrammes
Gravitational field strength, or g, is measured in newtons per kilogramme (N/kg).
Change in vertical height in metres (m) equals Δh.
The Greek letter "delta" (Δ) in physics stands for "change in.”
Gravitational Field Strength
On Earth, the gravitational field strength (g) is about 10 N/kg.
The Moon's surface experiences a weaker gravitational field than that of Earth.
This means that lifting a mass would be simpler on the Moon than it would be on Earth.
On the surface of gas giants like Jupiter and Saturn, the gravitational field intensity is stronger than it is on Earth.
This indicates that lifting a mass on the gas giants would be more difficult than on Earth.
1.3 Kinetic Energy
The definition of energy in the kinetic store of an object is the quantity of energy that an item possesses given its mass and speed.
This implies that the kinetic energy store of any moving object has energy.
The following formula can be used to determine kinetic energy.
KE = ½ x m x v²
Where:
J (Joules) is the unit of kinetic energy (KE).
m = the object's mass in kilogrammes (kg)
v is the object's speed in metres per second (m/s).
1.4 Closed Systems
A system is defined in physics as an item or collection of items
In physics, defining a system is a means of reducing the scope to include just those aspects that are pertinent to the observed condition.
Nothing happens when a system is in equilibrium because nothing changes.
Energy is transferred whenever a system changes.
For instance, one definition of a system is an apple on a table.
The fruit will remain in the same spot if nothing changes. The apple will fall if the table is removed. The fruit transfers energy as it falls.
Energy is measured in units of joules (J)
The definition of a closed system is a system in which the total energy within it has not changed
Consequently, the total energy in that system needs to stay constant. This is a result of the energy conversation.