Amino Acids and Protein Structure
Amino Acids
Amino Acid Overview and Types
Overview
Proteins are linear heteropolymers of alpha-amino acids
Amino acids share many features, differing only at the R substituent (group)
the alpha carbon is chiral when the R group is anything other than hydrogen (so anything other than glycine)
all chiral amino acids are optically active
Because of tetrahedral arrangement of bonding orbitals around the alpha-carbon, the four different groups can occupy two unique spatial arrangements and thus have 2 possible stereoisomers: L and D
L and D versions of an amino acids are enantiomers
nonsuperimposable mirror images
only L-amino acids are found in proteins
L-amino acids have their amine group on the left side within a Fischer projection.
Amino acids are classified based on their R groups (charge, H-bonding ability, and if they’re acid/basic)
Two main categories: Hydrophobic and Hydrophilic
Hydrophobic amino acids are non-polar (includes AA’s with alkyl/aliphatic and with aromatic R groups)
Hydrophilic amino acids are polar, includes neutral, acidic, and basic R-groups
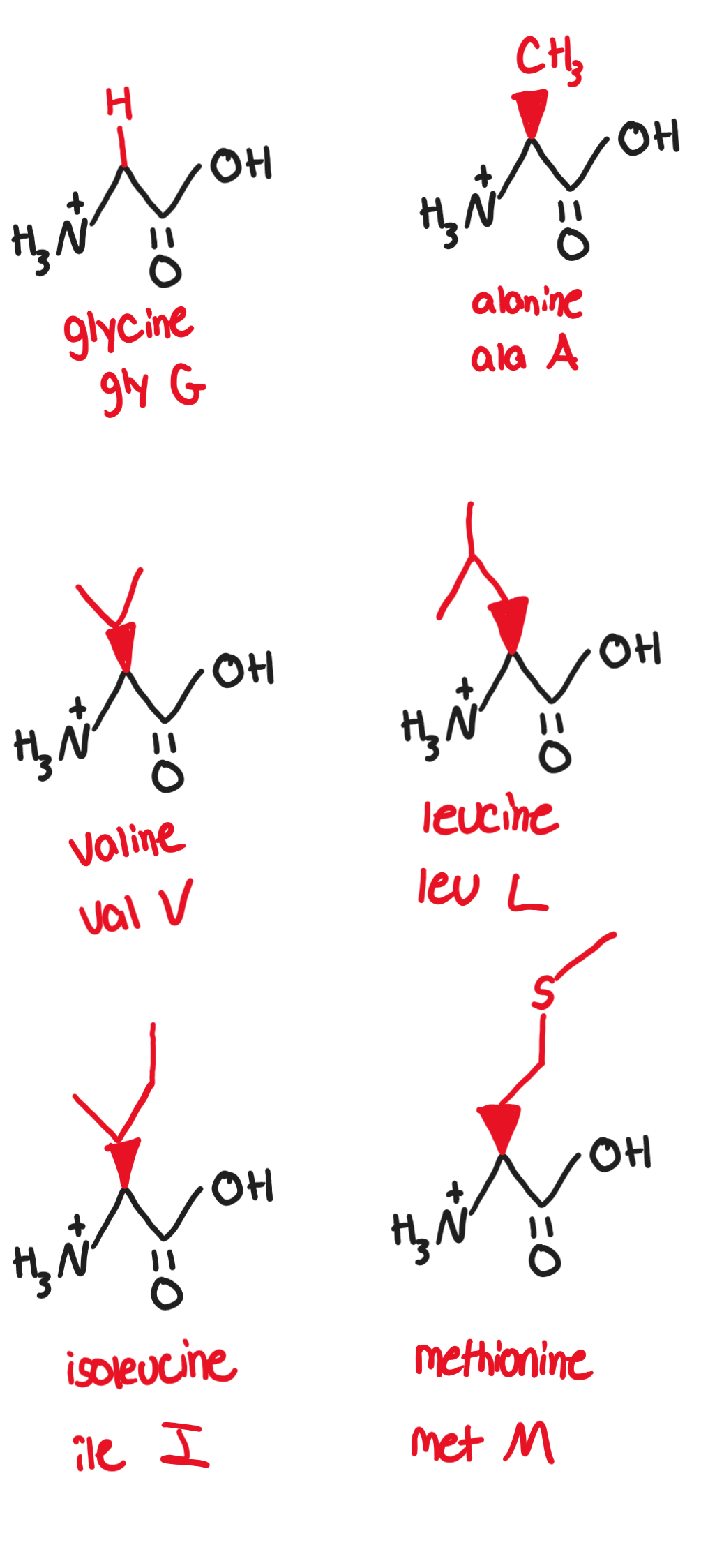
Hydrophobic, nonpolar, aliphatic/alkyl R groups:
Non-polar side chains consist mostly of hydrocarbons
any functional groups are uncharged at biological pH
Glycine, Gly, G (-H)
only achiral amino acid, has a slightly sweet taste
Alanine, Ala, A (-CH3)
canonical example of a simple. small, nonpolar amino acid
Proline, Pro, P (R-group is a ring, bonds with amino group twice)
can break up secondary structures due to the kink proline can cause
can be found within the turns of beta-pleated sheets
Valine, Val, V (-CH(CH3)2)
it substitution for glutamic acid in hemoglobin causes sickle-cell disease
contains isopropyl group
Leucine, Leu, L (-CH2CH(CH3)2)
essential amino acid (animals and humans cannot synthesize it ourselves so it must be obtained from diet)
Isoleucine, Ile, I (-CH(CH3)CH2CH3)
essential amino acid
Methionine, Met, M (-CH2CH2SCH3)
C-S bond makes molecule nonpolar, involved in DNA methylation and angiogenesis
essential amino acid found especially in eggs
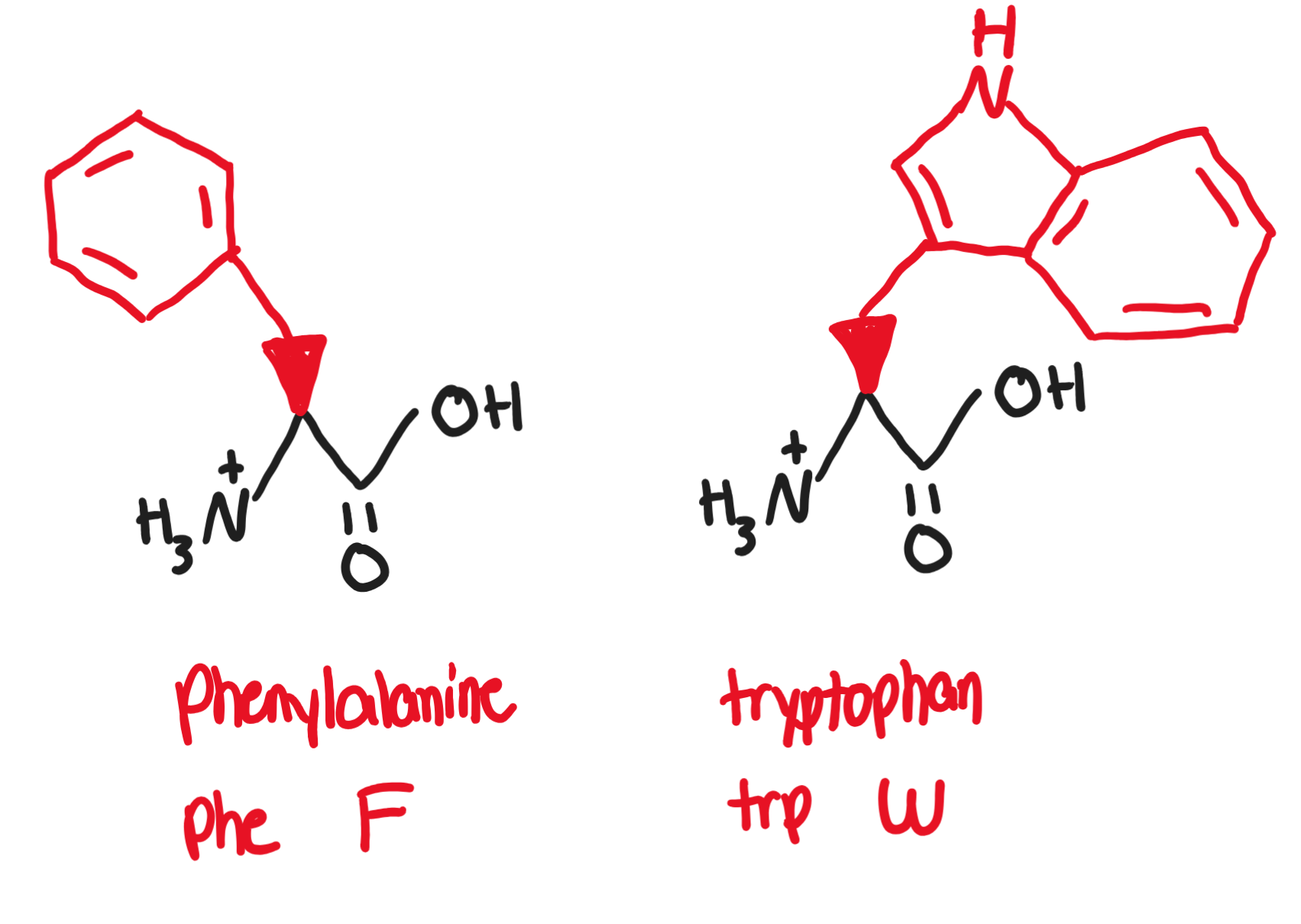
Hydrophobic, non-polar, aromatic R groups
Aromatic R-groups can absorb UV light
the higher the concentration of protein in a substance, the more UV light is absorbed
Phenylalanine, Phe, F (-CH2-benzene)
present in artificial sweetener aspartame
Tryptophan, Trp, W (two rings)
precursor of serotonin and melatonin
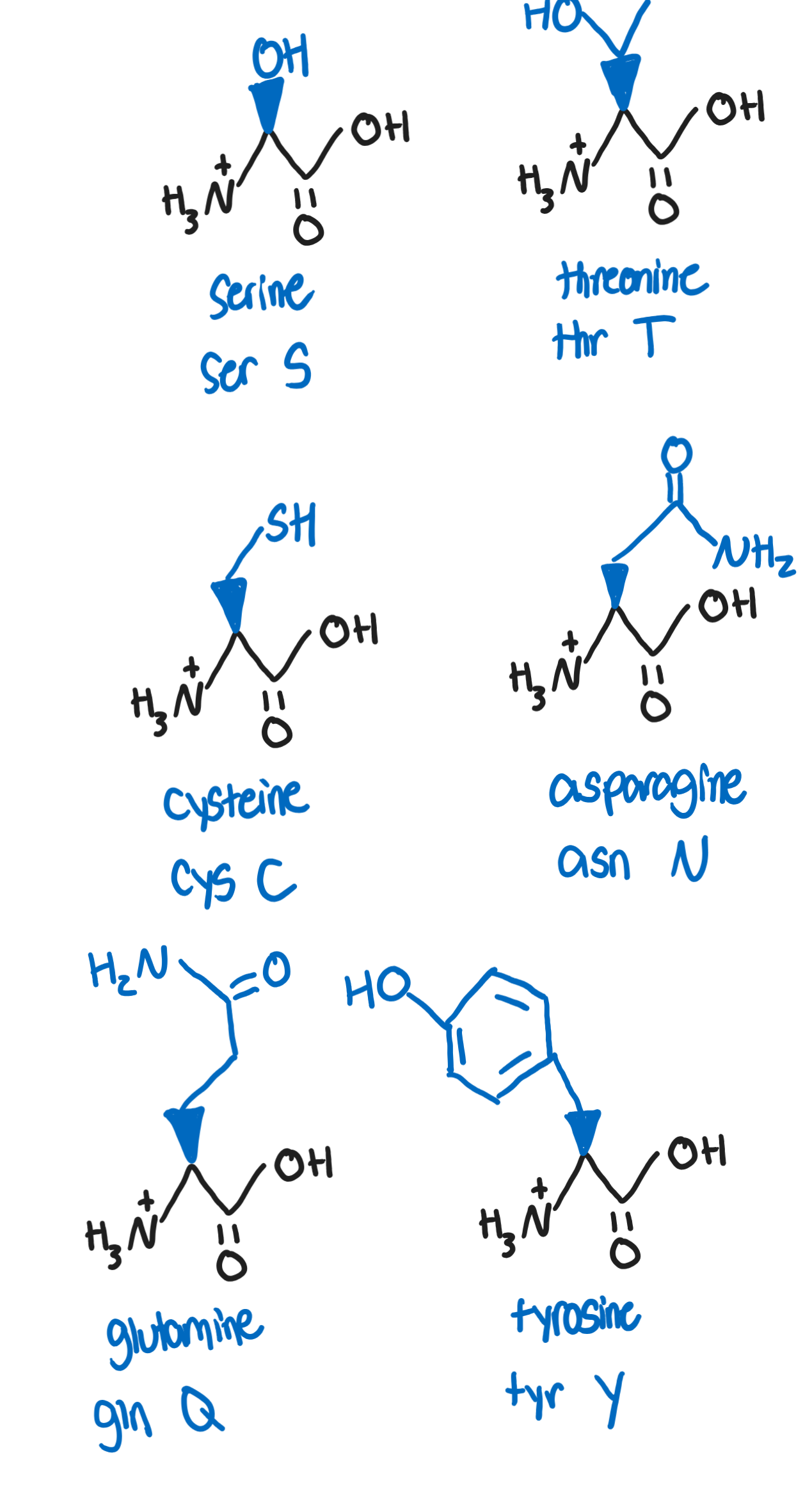
Hydrophilic, polar, neutral R groups
side chains are hydrophilic, thus on surface of proteins and are subjected to chemical modifications
can be oxidized or reduced, affecting conformation of protein
Serine, Ser, S (-CH2OH)
target for phosphorylation and other processes involved in post-translational modifications and signaling (due to OH group)
Threonine, Thr, T (-CH(OH)CH3)
target for phosphorylation due to OH groups
essential amino acid
Cysteine, Cys, C (-CH2SH)
can form covalent disulfide bridges (important in tertiary structures)
Asparagine, Asn, N (-CH2(CO)NH2)
reacts to reducing sugars (glucose and fructose) in baked and fried foods to form acrylamide, a potentially carcinogenic compound
Glutamine, Gln, Q (-CH2CH2(CO)NH2)
most common as a free-amino acid in human blood and is involved in a wide range of metabolic reactions
Tyrosine, Tyr, Y (CH2-benzene-OH)
can undergo post translational modifications and phosphorylation
aromatic
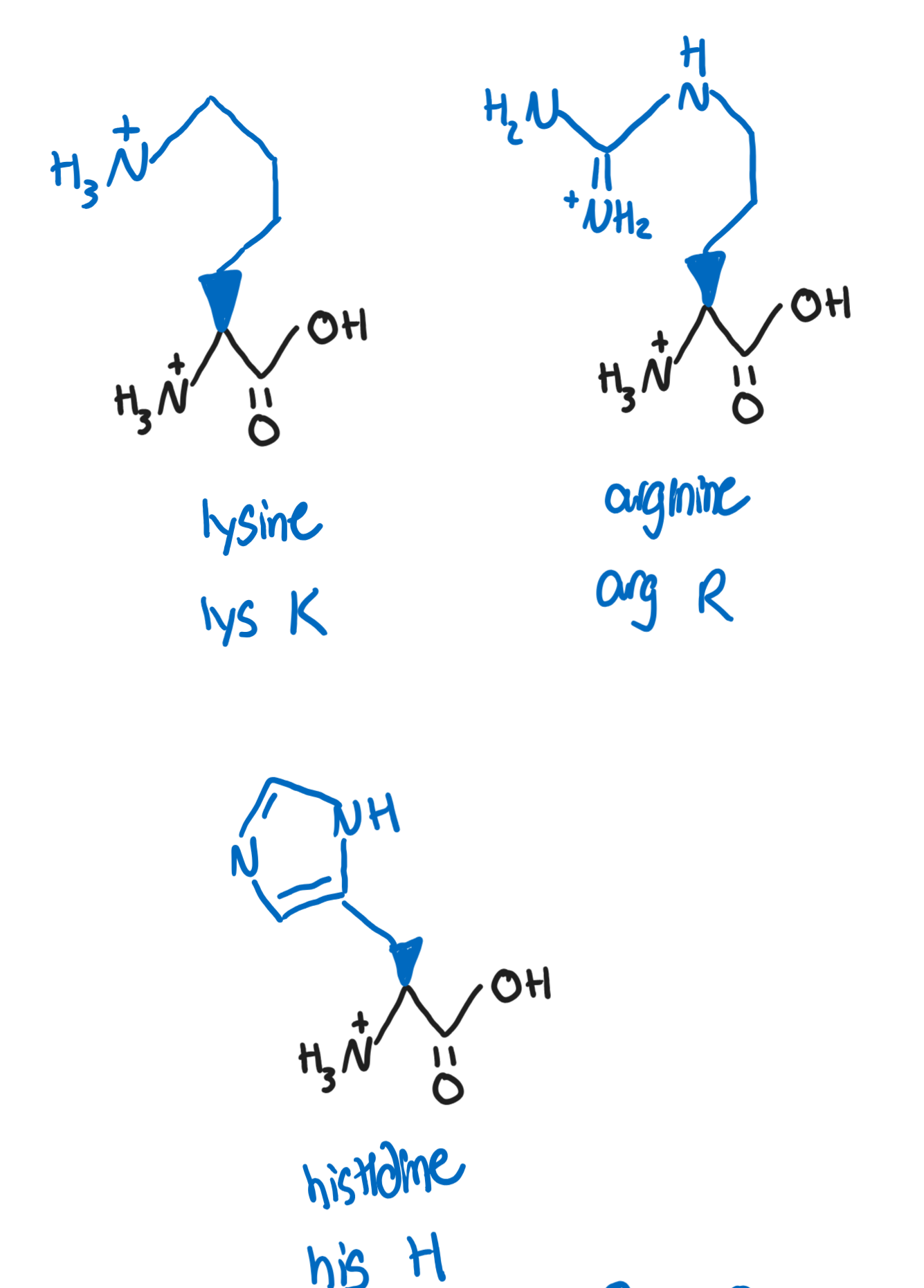
Hydrophilic, polar, basic R groups
Lysine, Lys, K (-(CH2)4NH3+)
the primary amine at the end of the chain is fairly reactive and is the target for many covalent modifications (methylation and acetylations)
Arginine, Arg, R (-NHC(NH2+)NH2)
plays a role in regulating blood pressure and other biomolecule synthesis
guanidino group can be protonated and can be resonance-stabilized (makes arginine the most basic of all amino acids)
Histidine, His, H (CH2-aromatic with N and NH2)
is deprotonated at physiological pH
can serve as a buffer in pHs slightly more acidic than physiological
Hydrophilic, polar, acidic R groups
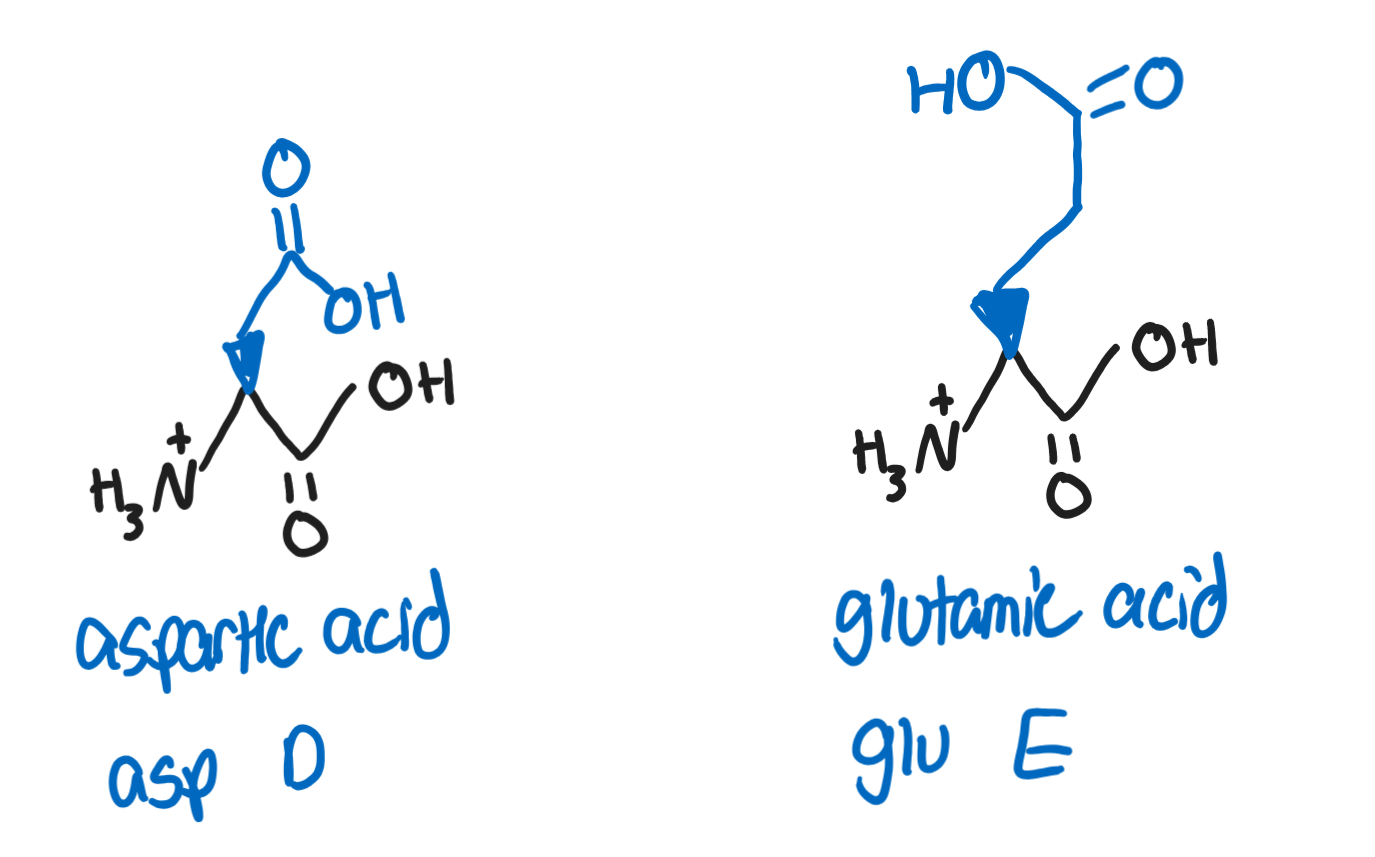
Aspartic Acid, Asp, D (-CH2COOH)
deprotonated form is called aspartate
a component of artificer sweetener aspartame
Glutamic Acid, Glu, E (-CH2CH2COOH)
deprotonated form is called glutamate
important as a structural amino acid in proteins and important in neurotransmitters
Acid/Bases and Titration Curve
Functional group properties vary at different pHs
in an aqueous solution, the carboxyl group acts as a weak acid and the amino group within a protein acts as a weak base
in very low pHs- both functional groups protonate (gain a proton or hydrogen) and become -COOH and -NH3+ respectively and have an overall charge of +1
in very high pHs- both functional groups deprotonate (lose a proton or hydrogen) and become -COO- and -NH2 and have an overall charge of -1
at physiological pH the carboxylic acid is deprotonated and the amine group is protonated, resulting in a net charge of 0
zwitterion: an amino acid with a deprotonated carboxylic group and a protonated amine group and contains a net charge 0
remember: at physiological pH- the net charge WITHOUT SIDE CHAIN CONSIDERATION is 0. We still need to figure out the charge with the variable chain included.
To find the charge of basic or acidic side chains- we need to use the Henderson-Hasselbalch equation:
pH=pKa+log([A-]/[HA])
pKa: the negative logarithm of the acid dissociation constant kA and is the measure of the tendency of a group to give up a proton
[A-] [HA]: the concentrations of deprotonated and protonated forms of acid respectively
when pKA=pH: the given functional group is half protonated and half-deprotonated (zwitterion) and is referred to as the isoelectric point of the amino acid (charge=0
pKa>pH: the quantity of protonated acid will increase
pKa<pH: the quantity of deprotonated acid will increase
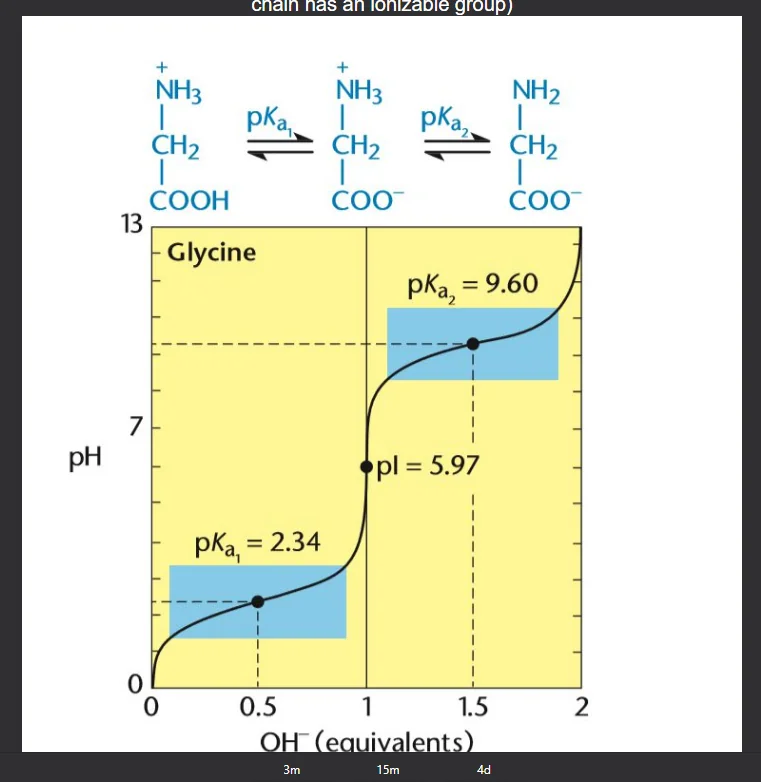 Titration Curve of Glycine
Titration Curve of Glycine
Finding pI= (pk1+pk2)/2
Amino Acid Reactions
Strecker synthesis
Gabriel Malonic Ester synthesis
Peptide bond formation
Peptide bonds are responsible for the linking of multiple amino acids together.
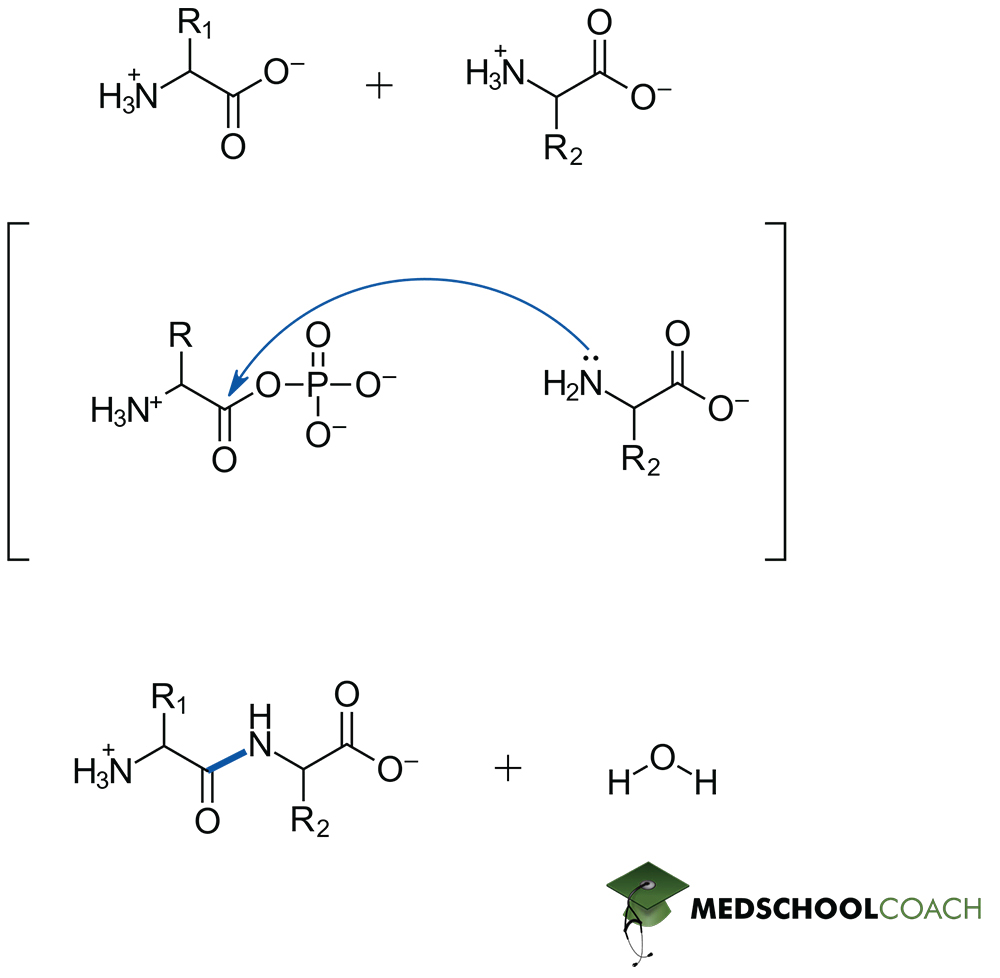
Peptide bonds bonds are formed by nucleophilic addition-elimination reaction between the carboxylic group of one AA and the amino group of the other AA, releasing water as a by-product.
also known as a dehydration reaction
peptide bonds are essentially amide bonds
Mechanism:
The nucleophilic amino group of the second amino acid attacks the electrophilic carbonyl carbon of the first amino acid.
A hydroxide is eliminated from the reaction and extracts a proton (hydrogen) from the amino group- resulting in the formation of water as the eliminate. The positive charge on nitrogen is also neutralized- thus a peptide bond is formed.
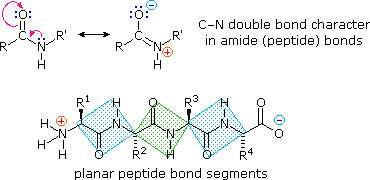
 NOTE:
NOTE:
The peptide bond is also just an amide bond.
The bond itself between the carbon and the nitrogen (highlighted above in blue) is stabilized by resonance delocalization- causing it to be a rigid and planar bond
this is due the movement of electrons from the nitrogen, to the carbon of the carbonyl group- causing the electrons on the double bonded carbonyl oxygen to turn into lone pairs.
Peptide Hydrolysis
Entails breaking a peptide bond within a polypeptide to form singular amino acid groups.
Breaking the peptides involves the assistance of strong acids or proteolytic enzymes:
Strong acid hydrolysis: uses a strong acid + heat to allow for non-specific cleavage
Proteolysis: allows for cleavage of a specific peptide bond by protease
Protein Structure
Dipeptide: 2 amino acids in a chain
Oligopeptides: chains made up to 20 amino acids
Polypeptides: consists of 2+ amino acids within a chain- but also more than oligopeptides
Protein sizes can be measured in molecular mass using units of kilodaltons (kDa)
1 kDA= 1g/mol
A peptide with a mass of 64kDa has a molecular weight of 64k grams per mole
the average size of proteins within human cells are estimated to be around 50kDa
Proteins are written from the N-terminus to the C-terminus and the order corresponds to how proteins are synthesized in the ribosome
Primary Structure
Primary structure is the linear chain of amino acid residues that makes up a protein
corresponds to a tRNA translation from a given strand of mRNA
The primary structure is held together by covalent peptide bonds that link amino acids together
the covalent peptide bonds allows for protein resilience to various environmental conditions (because of the resonance presented from the bonds)
Secondary Structure
Secondary structures refers to the structures that are formed by hydrogen bonding between the amine and carboxyl groups of neighboring amino acid residues
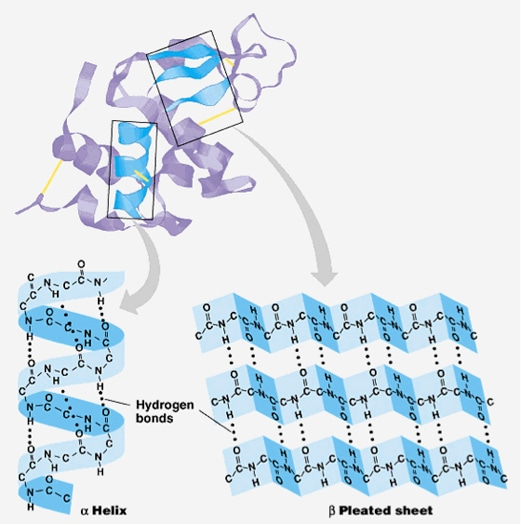
Forms common stable motifs such as alpha-helices and beta-pleated sheets
proline is well know for disrupting secondary structures by introducing proline kinks caused by it’s unique ringed sidechain that incorporates its amine group- but can be found within the inside turns of beta-sheets
alpha helices
helical backbone is held together by hydrogen bonding between the carbonyl of the nth amino acid and the amino group of the (n+4) amino acid
optimal H-O bond distance is 2.8 angstroms
right handed helix is the most common form with 3.6 residues per turn
favors small hydrophobic residues like alanine and leucine to form helices
formation can be affected by attractive or repulsive interactions between side chains that are 3-4 amino acids apart
alpha helices have large macroscopic dipole movement that can increase with helix length
![]()
beta-pleated sheets
hydrogen bonding happens mostly between neighboring peptide chains rather within the same polypeptide.
sheets exist in two forms: parallel and anti-parallel
parallel: hydrogen-bonded strands run in the same direction; resulting in bent H-bonds (weaker)
anti-parallel: hydrogen-bonded strands run in opposite directions, resulting in linear H-bonds (stronger)
Tertiary and Quaternary Structure
Tertiary Structure
Tertiary structures are defined as the three-dimension structures that result from interactions among side chains of the amino acid residues of a protein. Tertiary structures are heavily involved in protein folding and are commonly disrupted by denaturing agents.
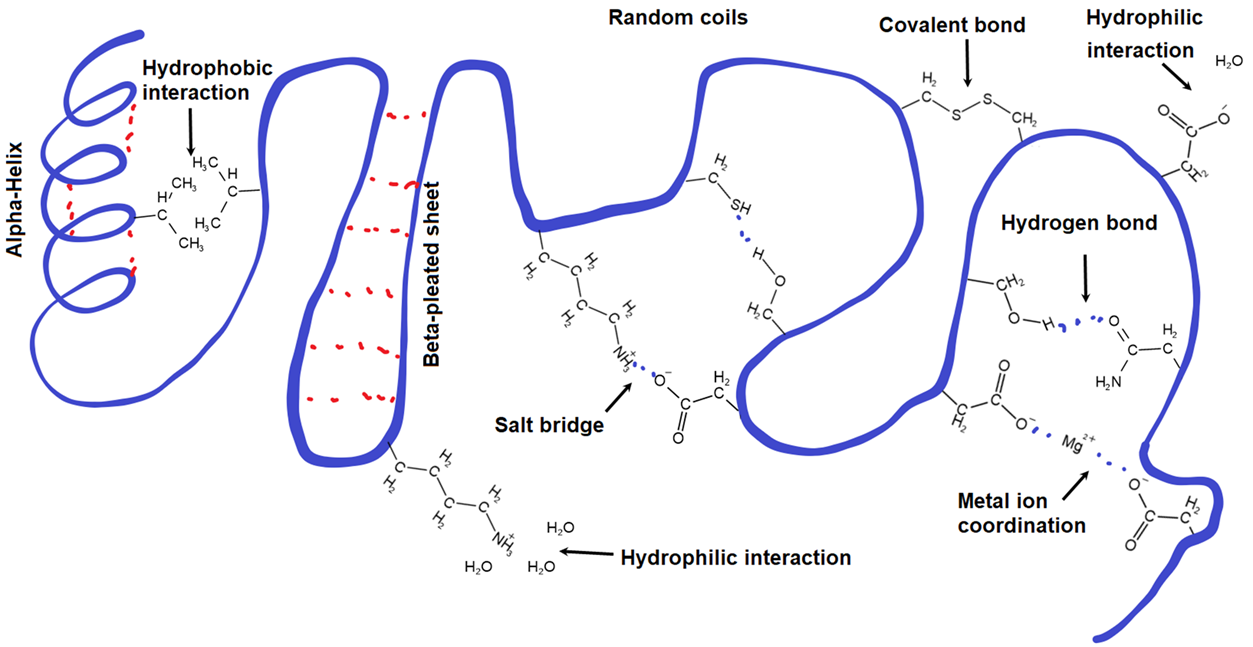
Non-covalent charge interactions
Hydrophobic interactions: non-covalent charge driven interactions that causes hydrophobic residues to cluster towards the inside of globular proteins
Hydrophilic interactions: noncovalent charge driven interactions that causes hydrophilic residues to aggregate on the external surface of proteins
Charged interactions
disulfide bridges: covalent bonds that form between when cysteine side chain residues are oxidized
can be broken apart with a reduction reaction in a ‘reducing environment’ (commonly with 2-mercaptoethanol)
salt bridges: bonds that happen with 2 charged residues interact
Quaternary Structure
Quaternary structures are larger structures generated by the assembly of protein subunits via non-covalent interactions and disulfide bonds. These structures are not present in all proteins.
The same bonds that occur in tertiary structure also happen in quaternary structure- the only difference is that these interactions occur between different subunits instead of within a singular subunit.