Chemistry Final
Wavelength and Frequency
Formulas:
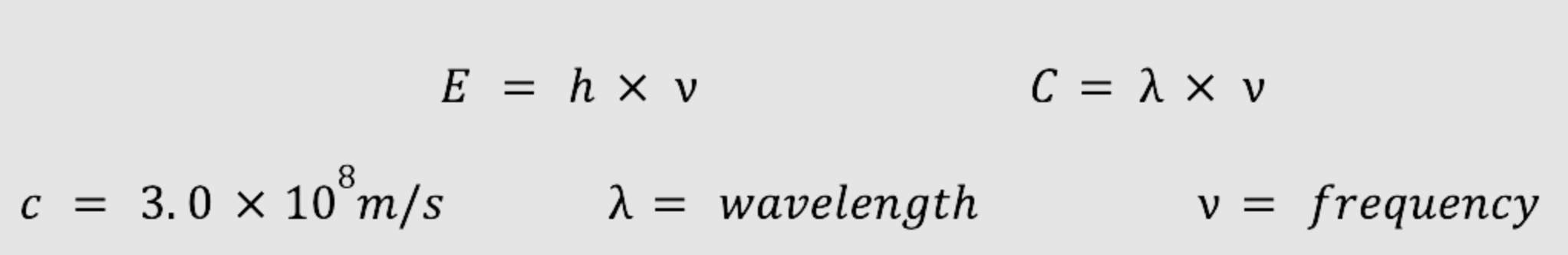 Practice problems:
Practice problems:
What is the frequency of a photon whose energy is 6.00 x 10-15 J? Identify the color of this radiation.
What is the energy of a photon whose frequency is 2.22 x 1014 s-1?
What is the wavelength of radiation whose frequency is 5.00 x 1012 MHz?
Principles/ Rules
Aufbau Principle: Lower energy levels must be filled before higher ones
Pauli Exclusion Principle: Electrons in the same orbital must have opposite spins
Hund’s Rule: One electron must be present before pairing
Periodic Trends
Periodic Table
Complete the following table based on the information given.
Isotopic Notation | Nucleotide Symbol | Atomic Number | Mass Number | Number of Protons | Number of Neutrons | Number of Electrons |
He-4 | 42He | 2 | 4 | 2 | 2 | |
Mg 2+-____ | 12 | 24 | ||||
Zn-65 | 30 | 30 | ||||
Br 1-- 80 | 35 | |||||
Al3+-_____ | 13 | 14 | ||||
C 4--_____ | 6 | 8 | ||||
Si-_____ | 29 | 14 |
