Module 1A: Biological Interactions
Module 1A consists of 5 topics, including:
The Molecules of Life - what are they and what do they have in common?
Revision orbitals and elements
Electronegativity and bond polarity
Covalent bonds
Types of Non-covalent bonds
Hydrogen bonds
Charge-charge interactions
Van der Waals interactions
Hydrophobic interactions
Required Reading:
Appling, Anthony-Cahill & Matthews. Biochemistry: Concepts and Connections. 2nd Ed. He
Chapter 2: The Chemical Foundations of Life: Weak Interactions in an Aqueous Environment
Topic 1: The Molecules of Life
Learning Objectives:
To be able to recall the major classes of macromolecules that are essential to life.
To be able to explain the properties of a covalent bonds
To be able to name the non covalent interactions.
To explain the role non covalent bonds have in nature and why they are so important to life.
1.0 Biological Macromolecules
Biological Macromolecules: are giant molecules made up of smaller organic molecule subunits. There are four major types:
Proteins
Nucleic Acid
Carbohydrates
Lipids
1.1 Biopolymers: Proteins, Nuclei Acids and Carbohydrates
Biopolymer: a large molecule made together by joining together prefabricated units, or monomers.
linked together via covalent bonds
Example:
Carbohydrate: Cellulose – composed of glucose monomers
Protein: Collagen – made up of amino acid monomers
Polypeptides = protein chains – formed by the linkage of amino acids through peptide bonds.
Nuclei Acids: are composed of nucleotide monomers
also called polynucleotides; assembled from combinations of the 20 amino acids
Of the aforementioned biological macromolecules, three of the classes ae biopolymers: carbohydrates, proteins and nucleic acids.
Lipids are not classified as polymers because they are not composed of repeating monomeric units; instead, they are made up of glycerol and fatty acids, which can vary in structure and composition.
Monomer: a single, simple molecule
Monomers of a given macromolecule are of limited diversity (hence why lipid is not a polymer), and are linked together, polymerised, by identical mechanisms.
Polymerisation process often includes condensation, removal of water molecule in joining reaction.
1.2 Lipids and Membranes
Lipids: are chemically diverse group of compounds that are classified together due to hydrocarbon-rich structures; enables them low solubility in aqueous environment
Major Function: serve as major structural element of the membranes
The types of lipids include:
Phospholipid – are amphipathic; hydrophobic and hydrophilic functional groups
When placed in water, readily form a bilayer; polar head groups, non-polar tails
contains both positive and negative charges
Triglycerols – are esters, formed by condensation between hydroxyl groups and three fatty acid molecules
Sterols – characterized by a four-ring core structure, which plays a crucial role in cell membrane fluidity and signaling
Cholesterol
2.0 Non-Covalent Interactions
Non-covalent bonds are important as they define the structure and function of biomolecules such as proteins, enzymes, nucleic acids, polysaccarides and their complexes.
Non-covalent bonds are:
Versatile and Flexible
Strong and Stable
2.1 The Nature of Non-Covalent Interactions
Non-Covalent Interactions: involve interactions with molecules that do not involve the sharing of electrons; allowing for temporary associations.
are fundamentally electrostatic; meaning, they arise from the attractions and repulsions between charged particles, which can include ions, polar molecules, and even temporary dipoles.
There are several types of non-covalent interactions:
Charge-Charge (Ionic)
Charge-Dipole
Charge-induced dipole
Dipole-induced dipole
Dispersion/ Van der Waals Interactions
Hydrogen bonds
2.1.1 Charge-Charge (Ionic) Interactions
Ionic Interactions: These are forces that occur between charged particles, where positively charged ions are attracted to negatively charged ions
2.1.2 Dipole and Induced Dipole Interactions
Dipole Moments: These occur when there is a separation of charge within a molecule, resulting in a positive end and a negative end, which can interact with other dipoles or induce a dipole in nearby neutral molecules.
these molecules with large dipole moments are highly polar
The strength of the interaction is influenced by the magnitude of the dipole moment and the distance between the interacting molecules; closer = stronger interactions.
Charge-dipole Interaction: a polar molecule, in an aqueous environmenr, can be attracted to a nearby ion
Dipole-Dipole Interaction: A polar molecule, in aqueous environment, is attracted to another polar molecule
Induced-dipole Interactions: These occur when a nonpolar molecule becomes polarized due to the presence of a nearby polar molecule, leading to temporary attractions.
Charged-induced Dipole Interaction: charged particle induces dipole in a polarized molecule and results in an attractive force between the molecules
Dipole-Induced Dipole Interaction: occurs when a polar molecule – permanent dipole – induces a dipole in a nonpolar molecule, resulting in an attraction between the two molecules.
Note: Two molecules – both of which have neither a net charge nor permenant dipole moment – can attract one another if close enough due to the formation of temporary dipoles, highlighting the significance of induced-dipole interactions in various biological processes.
the distribution of electronic charge fluctuates; hence fluctuations can result in an area of one molecule becoming temporarily more positive or negative
creating a temporary dipole that can attract another nearby nonpolar molecule → Van der Waals interactions
2.1.3 Van der Waals Interactions
Van der Waals Interactions: weak, non-covalent molecules that occur when they come close enough that their electron clouds begin to overlap, creating temporary dipoles that induce attractive force between them
There are two types of Van Der Wall Interactions:
Dipole-Induced Dipole Interactions
London Dispersion Forces: weakest type; non-polar molecules experience temporary shifts in electron density, leading to the formation of transient dipoles that can attract other molecules.
Note: Van der Waals Interactions is a broad term used to describe …
2.1.4 Hydrogen Bonds
Hydrogen Bond Interaction: is an interaction between a hydrogen atom covalently bonded to a highly electronegative atom, and another electronegative atom, resulting in a strong dipole-dipole attraction that plays a crucial role in the properties of water and the structure of proteins.
Nitrogen (N), Oxygen (O), and Fluorine (F) are the primary electronegative atoms involved in hydrogen bonding, significantly influencing molecular interactions and stability in biological systems.
Hydrogen-bond donor: The atom that is covalently bonded to the hydrogen
Is dependent on it’s electronegativity
Hydrogen-bond acceptor: the atom with the lone pair of electrons that attracts the hydrogen atom
AAre also highly directional; plays a crucial role in organising a regular biochemical structure such as the a-helix in proteins
2.2 Why are Non-Covalent Bonds Important?
Non-covalent bonds are critically important to biomolecular structure, stability and function.
3.0 Covalent Bonds (REVIST)
Covalent Bonds: strong interactions formed when two atoms share electrons, allowing for the stability and integrity of biological molecules.
There are two types:
Non-polar covalent bonds: formed when two atoms share electrons equally, resulting in no partial charges and a balanced distribution of electrical charge.
Polar covalent bonds: formed when two atoms share electrons unequally, leading to a partial positive charge on one atom and a partial negative charge on the other, which creates a dipole moment essential for many biological processes.
3.1 Properties of Covalent Bonds
Strength: Covalent bonds are generally strong and require significant energy to break, which contributes to the stability of molecules in biological systems.
Directionality: The orientation of covalent bonds between atoms influences the overall shape and function of the resulting molecules, playing a crucial role in biochemical interactions.
Topic 2: Revision of Orbitals and Elements
Learning Objectives:
Be able to explain what the common elements essential life are and describe their broad functions.
Be able to draw the orbital structure of H,C,N, O S, P and know the maximum number of bonds to each element
1.0 The Chemical Elements of Cells and Organisms
The most common elements essential to life include: carbon (C), hydrogen (H), oxygen (O), nitrogen (N), phosphorus (P), and sulfur (S).
Carbon (C) Function: serves as the backbone for organic molecules, allowing for the formation of complex structures and various functional groups.
Hydrogen (H) Function: plays a crucial role in the formation of water and organic compounds, participating in various biochemical reactions and maintaining pH balance in cells.
Oxygen (O) Function: essential for cellular respiration, oxygen acts as a final electron acceptor in the electron transport chain, enabling the production of ATP, the energy currency of the cell.
Nitrogen (N) Function: a key component of amino acids and nucleic acids, nitrogen is vital for protein synthesis and the formation of DNA and RNA, thus supporting genetic information and cellular functions.
Phosphorus (P) Function: critical for the formation of ATP and nucleic acids, phosphorus plays a significant role in energy transfer and storage, as well as in the structural integrity of cell membranes through phospholipids.
Sulfur (S) Function: important for the synthesis of certain amino acids, sulfur contributes to the formation of proteins and enzymes, and is also a component of coenzymes that facilitate various biochemical reactions.
Note: All of the aforementioned elements are found in the major classes of macromolecules
Topic 3: Electronegativity and bond polarity
Learning Objectives:
Be able to explain what electronegativity is and to be able to understand how this relates to the polarity of a bond.
Be able to draw the water molecule, and explain its structure through electronegativity
Recognise which polar bonds are important in biochemistry
Be able to explain why polar molecules are soluble in water
1.0 Electronegativity and Polarity
1.1 Electronegativity
Electronegativity of Atoms: refers to the atoms tendency to attract electrons
Fluorine (F) is the most electronegative element
Considering the elements most essential to life and biological processes – carbon, hydrogen, oxygen, nitrogen, phosphorus and sulfur – electronegativity tends vary
Highest to lowest: Oxygen → Nitrogen → Sulfur → Carbon → Hydrogen → Phosphorus.
Is measured via Pauling-scale
1.2 Bond Polarity
Bond Polarity: refers to the distribution of electrical charge around the atoms, chemical groups or molecules
Polar Bonds: are formed when there is a significant difference in electronegativity between the two atoms involved, resulting in a partial positive charge on one atom and a partial negative charge on the other.
Non-polar Bonds: occur when the electronegativity difference between the two atoms is negligible, leading to an equal sharing of electrons and no permanent dipole moment.
1.3 Electronegativity and Bond Polarity – Water Example
Water is a polar molecule as oxygen and hydrogen have different electronegativities – oxygen having a higher electronegativity than hydrogen.
The hydrogen atoms = partial positive charge; oxygen atom = partial negative charge, leading to the overall polarity of the water molecule.
Recall: Oxygen is greedy, and has a greater share of the electronegativity in the bond
1.3.1 Electronegativity and Bond Polarity – Non-polar Comparison
Methanethiol is a non-polar molecule due to its symmetrical structure; the difference in electronegativity is small
electrons are shared equally
1.4 Important Polar Bonds
O–H : O (δ-)← H (δ+) - This polar bond is significant in many biological molecules, contributing to their solubility and reactivity in aqueous environments.
N–H: N (δ-)← H (δ+) - This polar bond also plays a crucial role in the structure and function of proteins, influencing hydrogen bonding and molecular interactions.
C–N: C (δ+) → N (δ-) - This polar bond is essential in the formation of amino acids and nucleotides, affecting the stability and interactions of various biomolecules.
C–O: C (δ+) → O (δ-) - This polar bond is important in carbohydrates, affecting their reactivity and interactions with other biomolecules, as well as their role in energy storage and structural functions.
S–H:
Topic 4: Covalent Bonds
Learning Objectives:
Be able to recognise polar and non polar covalent bonds and explain their properties.
1.0 Covalent Bonds – They Like to Share
Many macromolecules of life are polymers of monomers held together by covalent bonds.
Covalent bonds are formed by the sharing of electrons
Strength of a covalent bond 100-1000kj.mol-1; depends on the types of atoms involved in the bonds
Covalent bonds can have the following properties:
Covalent bonds are strong
Covalent bonds have a defined length
Covalent bonds have a defined direction
1.1 Covalent Bonds – Polarity
Covalent bonds can be polar or non-polar.
1.1.2 Polar
Unequal sharing of electrons by atoms.
as electronegativity increases, the electron pair in a bond is more closely associated with one nucleus than the other
If electronegativity difference is between 0.4-1.7 = polar
If electronegativity difference > 1.7 = the bond is ionic
1.1.3 Non-polar
Non-polar bonds occur when atoms equally share electron pairs.
Only identical atoms can form non-polar bonds, as their electronegativities are the same, resulting in an equal distribution of electron density.
electronegativity difference <0.4 = non-polar
Topic 5: Types of Non-covalent Interactions
Learning Objectives:
Understand and be able to explain why non covalent interactions are electrostatic
Be able to compare and contrast the properties of non covalent interactions to covalent bonds
Be able to compare the relative strengths of non covalent interactions and covalent bonds
1.0 Molecular Interactions
Molecular Interactions: are attractive or repulsive forces between molecules and between non-bonded atoms.
are electrostatic
also known as non-covalent interactions/intermolecular interactions, ect.
Non-covalent interactions give macromolecules:
Versatility and Flexibility: because they can be easily be broken and remade; weak 9< 30 kJ/mol)
are approximately 10-100 times weaker
Strength and Stability: one bond alone is not strong enough to hold these large structures together but because they are together in networks that comprise thousands of bonds they are strong and stable
1.1 Types of Non-Covalent Interactions
Hydrogen Bonds (Dipole-dipole interaction): involves polar bonds and partial charges on atoms
Electrostatic Interactions (Ion pair, salt bridges, ionic bonds): attraction of opposite charged groups (or repulsion of like charges)
Van der Waals Interactions: weak attractive forces between permanent or inducible dipoles; numerous and very important for macromolecule structure and interactions
Hydrophobic Interactions: association of non-polar groups with most energy attributed to the exclusion of water (increased entropy of released water molecules) which is favourable
Topic 5a: Hydrogen Bonds
Learning Objectives:
Be able to explain what a hydrogen bond is and its important properties.
Be able to show what the important biological hydrogen bonds are in nature
Be able to recognise and draw hydrogen bond donors and acceptors
Be able to show how hydrogen bonds stabilise proteins, DNA
Be able to explain and draw how water hydrogen bonds to itself
1.0 Hydrogen Bonds
Hydrogen bonds play a major role in stabilising the 3D structure of important biological macromolecules such as proteins, polysaccharides and nucleic acids.
Hydrogen bonds are amongst the strongest most specific non-covalent interactions
Hydrogen bonds play a major role in stabilising the three-dimensional structure of proteins and other important biological macromolecules
are a dipole-dipole interaction
are weak bonds (2-21 kj/mol)
The most stable hydrogen bonds are close to linear
They are directional
1.1 Hydrogen Bonds in Biological Systems
Play a crucial role in stabilizing the structures of proteins and nucleic acids.
Contribute to the unique properties of water, which is essential for life.
1.1.1 Hydrogen Bond Donors and Acceptors
Hydrogen bond donors: molecules that provide a hydrogen atom involved in the bond, typically containing electronegative atoms
oxygen or nitrogen.
Hydroxyl groups
amines/imines
Hydrogen bond acceptors: molecules that contain lone pairs of electrons capable of forming hydrogen bonds, often found in electronegative atoms like oxygen, nitrogen, or fluorine.
Keto groups
Amines
Imines
Hydroxyl groups
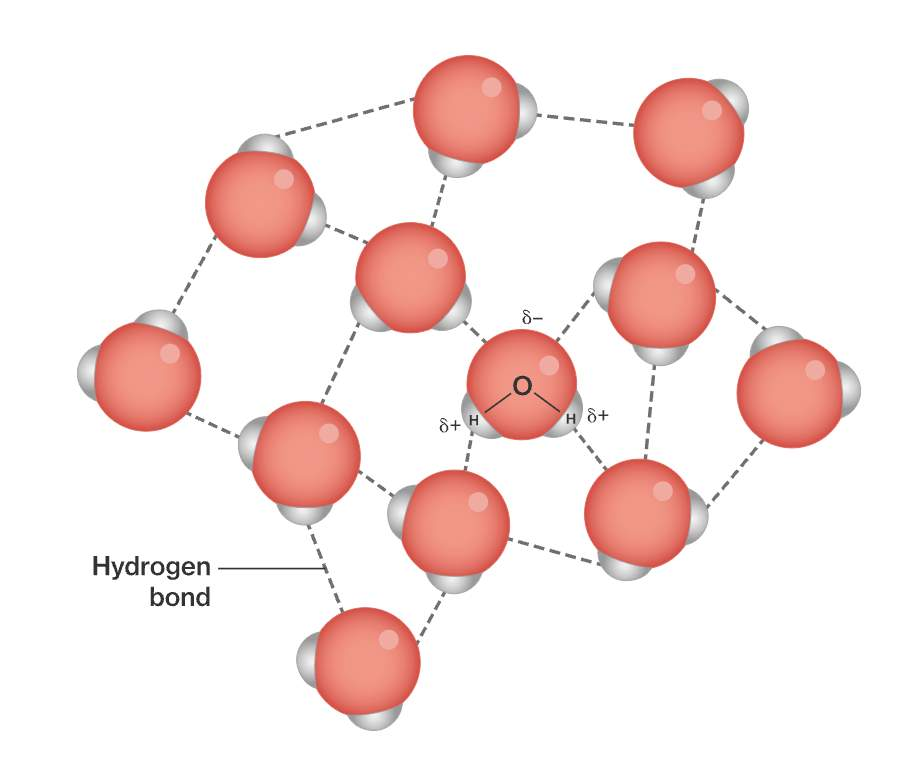
Water: A Hydrogen Bond Donor and Acceptor
Due to it’s polar structure, water is capable of forming hydrogen bonds with other molecules
Topic 5b: Charge-Charge Interactions
Learning Objectives:
Be able to describe the properties and draw the molecular structure of a charge charge interaction
Know and be able to describe other ions in biological solutions and the affect that these ions can have on charge charge interactions properties.
Be able to explain how ions can stabilise the structure of nucleic acids
1.0 Charge-Charge Interactions
Charge-charge interactions are another type of electrostatic interaction.
Also called ion pairs or salt bridges or ionic interactions
Electrostatic interactions: occurred between charged, positive and negative, ions
1.1 Salt Bridges in Biological Systems
Favourable electrostatic interactions between paired anionic and cationic amino acid side-chains are common in proteins
Salt bridges are formed when the charged group of a cationic amino acid is between 3.0 to 5.0
charged groups are generally linked via hydrogen bonds
e