Microbiology Chapter 5: Metabolism
Metabolism - sum off all an organism’s chemical reaction
Metabolic reactions are enzyme catalyzed which means that the product of one reaction is the reactant for the next reaction.
Chemical reaction are either:
Energy-releasing (exergonic)
Energy-requiring (endergonic)
What links these processes is the different forms of ATP - made or used
ATP - performs much of the cellular work
ATP hydrolysis - energy-releasing
ATP formation - energy requiring
When a negatively charged molecule is broken, it releases energy because it’s unstable and contains a lot of potential energy
ATP on the left side (reactant) is energy-releasing. ADP is one the left side with ATP on the right is energy-requiring.
Catabolism - taking larger, complex molecules and breaking it down to simpler molecules
energy is released
ATP formation - catabolism releases the energy needed for the ATP formation
Anabolism - taking simpler molecules and creating larger, complex organic molecules
energy is used
ATP hydrolysis because hydrolysis releases energy and the energy released is taken by anabolism process
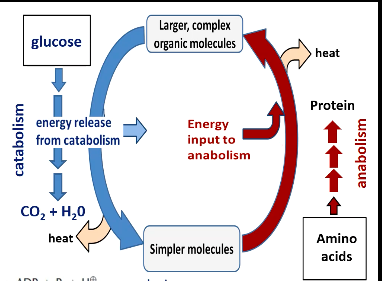
Heat loss accompanies all energy transfer processes
The energy from catabolism is used to form ATP. The ATP created from catabolism is broken down and hydrolyzes and is used to aid in creating simpler molecules into larger, complex molecules
Energy is stored in the chemical bonds
Molecules are broken down in order to capture energy in the form of electrons. EX: Glucose in Catabolism
Glucose is broken down in two stages.
Glycolysis where it’s broken down into two pyruvates
Hydrogen atoms is where energy is captured via electrons
Cellular respiration where pyruvate is broken down into CO2
Energy is again captured via electrons as Hydrogen atoms
EX: CO2 in Anabolism
Add electrons to CO2 which requires energy
Oxidation - losing electrons
Reduction - gain of electrons
OILRIG - oxidation is loss, reduction is gain
When looking at which was reduced and oxidized, look at hydrogen atoms and see how they’ve moved
Role of electron carriers: capture and transfer electrons via H atoms
EX: NAD/NADH & FAD/FADH2
Energy released by redox reactions are captured to form ATP via phosphorylation of ADP: ADP + P1 + Energy → ATP
Substrate-level phosphorylation: (fermentation, respiration)
Phosphorylated substrate can serve as the phosphate to ADP to create ATP. You need ADP and Phosphate to create ATP
Oxidative Phosphorylation (respiration)
Photophosphorylation (photosynthesis)
Both oxidative and photophosphorylation requires electron transfer chains and utilizes process of chemiosmosis to form ATPs
involves use of proton gradient and ATP synthase
Photophosphorylation driven by light
Respiration - employes electron transfer chain & terminal electron acceptor; can be aerobic w/ O2 as terminal acceptor or anaerobic with NO3- or other terminal acceptor
Glycolysis where glucose is broken down into pyruvic acid → Pyruvic acid is produced into Acetyl-CoA → Krebs Cycle creates ATP and CO2 → Electron Transport → H2O
Fermentation - anaerobic process, incomplete oxidation of carbohydrate
only takes product of glycolysis or pyruvate acid and breaks it down to acetic acid or lactic acid, etc. Small organic acids
Fermentation is usually considered incomplete because there is still a lot of energy in the produces of Fermentation
Fermentation is only producing ATP because of glycolysis
Glycolysis breaks down glucose into pyruvic acid → pyruvic acid and NADH is used to form fermentation end products
More ATP is created by respiration than fermentation
INSERT PICTURE OF RESPIRATION AND FERMENTATION PROCESS HERE
Glycolysis - oxidation of glucose to pyruvic acid, is usually the first stage in carbohydrate catabolism
First part is energy investment
The 6 carbon in Glucose is broken down by two ATP into two 3 carbon molecules with a phosphate group.
Second part is Energy Harvest
NADH is created
substrate phosphorylation occurs from the phosphate attached to the carbon molecules and combines with the ADP which then creates ATP and 2 pyruvates
creates 4 ATP and 2 NADH
Entner-Doudoroff pathway: sugar acids catabolism pathway; found in Gram-negative bacteria
Pentose-phosphate shunt: produce pentose sugars used for biosynthesis of:
Aromatic amino acids
Nucleotides
can also form pyruvate
Acetyl CoA Formation and the Krebs Cycle:
Acetyl CoA
Pyruvate oxidation to acetyl-CoA
decarboxylation, NADH formed
Two acetyl CoA is created from the two pyruvates
NADH is created and CO2
Krebs cycle (citric acid)
Glucose oxidation is finalized (converted to CO2)
3 NADH, 1 FADH, and 1 ATP per acetyl CoA → 2 Acetyl CoA leads to 6 NADH, 2 FADH, and 2 ATP
End of krebs cycle is when glucose is finally fully oxidized
Processes in the Krebs Cycle serve both catabolism and anabolism
Central point in metabolism
Electron Transport System:
Most of the energy extracted from glucose is contained in the reduced electron carriers, NADH and FADH2
The NADH and FADH2 accumulated would go down the electron transport chain
Components in an electron transport chain is in a membrane
Ends in oxygen if it’s aerobic metabolism
There’s a progressive loss of energy as it flows down because energy transfer is being used to pump out protons out.
The molecules need to keep providing it electrons to keep the electron transfer train going.
Oxygen has a high affinity to such electrons. It’s important to have a molecule like that in order to keep the electrons flowing towards it.
Chemiosmotic Mechanism
Has nothing to do with substrate phosphorylation. Involves the oxidative phosphorylation.
Creating a gradient. Protons are pumped via carriers in the electron transport chain using the energy from electron transfer.
Excess of H+ on one side of the membrane
electrochemical gradient
positive charge is attracted to the negative charge on the inside
Proton motive force
Both of the forces that want to bring the positive protons inside: charge and concentration
ATP synthase allows for energy to be harnessed from proton motive force because energy is released as positive protons move through the membrane towards the negative or down the gradient
powered by electron transport chain
More ATP is produced with chemiosmotic than substrate phosphorylation
Chemiosmosis is occuring in the cell membrane
Substrate-level phosphorylation creates 4 ATPs
Oxidative phosphorylation creates 10 NADH and 2 FADH2
3 ATP per NADH oxidized
2 ATP per FADH oxidized
Total: 30 ATP and 4 ATP
Anaerobic Respiration - The final electron is an inorganic substance other than oxygen
less yield than aerobic respiration
Substrate phosphorylation: glycolysis, Krebs
Oxidative phosphorylation: Glycolysis, Acetyl CoA, Krebs