6.3.1(c) recycling within ecosystems
spec points:
To include the role of decomposers and the roles of microorganisms in recycling nitrogen within ecosystems (including Nitrosomonas, Nitrobacter, Azotobacter and Rhizobium)
the importance of the carbon cycle to include the role of organisms (decomposition, respiration and photosynthesis) and physical and chemical effects in the cycling of carbon within ecosystems.
nitrogen cycle
nitrogen: essential component of metabolism, reacquired for synthesis of proteins and nucleic acids
cannot be used in gas form, to be of any use to plants, needs to be fixed in either form of ammonium ions/nitrates
key processes
Saprobiotic nutrition,
Ammonification,
Nitrification,
Nitrogen fixation
Denitrification.
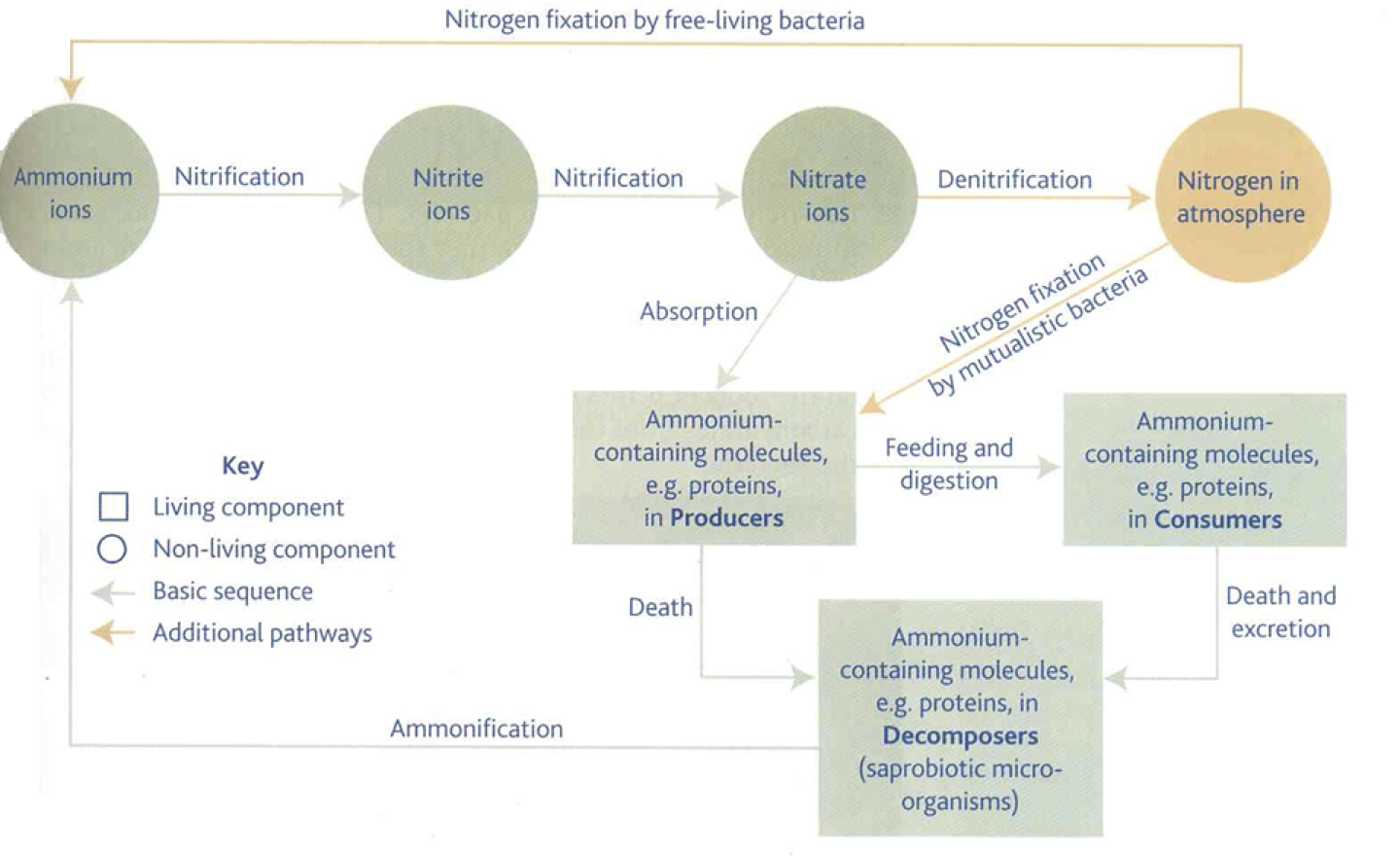
1. nitrogen fixation
Nitrogen gas ➔ nitrogen-containing compounds
nitrogen first fixed by bacteria (Rhizobium) which live in root nodules of leguminous plants e.g. pea plants. Azotobacter + Rhizobium bacteria contain nitrogenase enzymes to convert nitrogen and oxygen into ammonia. bacteria have mutualistic relationship with plant where they exchange fixed nitrogen for glucose
Azotobacter (Free-living bacteria ) – produce ammonia from nitrogen gas. Make amino acids. Release them when they die.
Mutualistic bacteria – live in root nodules in peas and beans. Obtain carbohydrates from plants and plants get amino acids from bacteria.
2. ammonification
anaerobic conditions (maintained with use of special oxygen-absorbing proteins) enable nitrogen reductase to reduce nitrogen gas to ammonium ions. Ammonium ions subsequently released by bacteria in putrefaction of proteins from dead organic matter.
Production of ammonia from organic compounds e.g. urea, proteins, nucleic acids.
Saprobiotic microorganisms (bacteria & fungi) feed on these to release ammonia into the soil
3. nitrification
Some microorganisms get energy from reactions involving inorganic ions (chemotrophic bacteria (Nitrosomonas) oxidise Ammonium ions ➔ nitrite ions (NO2-))
This is an oxidation & releases energy
Nitrobacter subsequently oxidise nitrites to nitrates in the presence of oxygen
Nitrite ions ➔ Nitrate Ions (NO3-)
This requires oxygen – occurs in soil with air pockets
e.g. aerated, well-drained soil
4. denitrification
Plants absorb nitrates from soil for nucleotide synthesis
In anaerobic conditions, denitrifying bacteria convert nitrates back into oxygen gas for respiration. Nitrogen gas and nitrous oxide are produced in the process
Occurs when soils become waterlogged – short of O2
Anaerobic bacteria carry out denitrification
Soil nitrates ➔ nitrogen in the atmosphere
Reduced Nitrogen compounds available to plants – for land to be productive it must be kept well aerated.

carbon cycle
carbon: component of all organic molecules and as such recycled through environment by processes of photosynthesis, feeding, respiration and decomposition
role of organisms in carbon cycle
carbon is constantly being recycled around the biospher so that number of carbon atoms in the biosphere is essentially constant; carbon atoms merely swap from one compound to another by various processes in CC
stored in many forms
atmosphere (CO2)
sedimentary rocks
fossil fuels like coal, oil and gas; coal is almost pure carbon
soil + other organic matter
vegetation (cellulose)
dissolved in oceans (CO2)
photosynthesis
Autotrophs use energy of sunlight to 'fix' carbon dioxide, turning carbon into sugars + other organic molecules - removing carbon from atmosphere
Calvin cycle is where CO2 is fixed, by enzyme Rubisco (carboxylates RuBP)
Terrestrial plants use gaseous CO2 directly from air
Aquatic organisms use CO2 dissolved in water
As much CO2 is fixed from ocean microorganisms, as from terrestrial plants
sedimentation
Saprobionts don’t fully decompose plants that die; their bodies form layers of sediment - can accumulate over millions of years, locking carbon into ground
sediment = store of energy, can form fossil fuels like peat + coal
aquatic organisms that die also form sediments on sea bed; can go on to form other fossil fuels e.g. oil, gas
shells + other calcium-containing body parts can form sedimentary rocks e.g. limestone
existence of life forms over billions of years has shaped biosphere, in that their remains are still being recycled
respiration
All life forms respire, including autotrophs
Heterotrophs rely on respiration for all their energy needs
Respiration puts CO2 into the atmosphere, in the opposite direction to photosynthesis
CO2 is released in the Link Reaction and the Krebs Cycle of aerobic respiration
Anaerobic respiration also releases CO2 into the atmosphere, via fermentation by yeast, moulds and bacteria
feeding
Carbon passed from autotroph to heterotroph during feeding
Carbon is also passed from primary consumer to secondary consumer
Biomass transfer always includes the transfer of carbon, the main element in biomass
decay & decomposition
dead plants + animals fed upon by detritivores + decayed by saprophytes
Releasing carbon into surroundings
Supplying carbon to detritivores
Supplying carbon to saprophytes
Waste matter e.g. faeces, urine used by decaying saprobionts
Such processes can release CO2 back into the air

burning fossil fuels
since mid 19th century, humans extracted + burned increasing amounts of fossil fuels
CO2 is being returned to atmosphere faster than it’s absorbed by plants + aquatic producers
CO2 level in atmosphere is approx. double that of 800,000 years ago
warmer temp. = less CO2 can be dissolved in oceans, so is released into air
has caused dramatic climate change + affected many other species, mainly through changing habitats