VSEPR & Molecular Geometry
6 electron domains - sp3d2
5 electron domains - sp3d
4 electron domains - sp3
3 electron domains - sp2
2 electron domains - sp
6 bonds, 0 lone pairs - Octahedral, 90
5 bonds, 1 lone pair - Square Pyramid, <90
4 bonds, 2 lone pairs - Square Planar, 90
3 bonds, 3 lone pairs - T-shaped, 90
2 bonds, 4 lone pairs - Linear, 180 5 bonds,
0 lone pairs - Trigonal bipyramidal, 90 &120
4 bonds, 1 lone pair - Seesaw, <90
3 bonds, 2 lone pairs - T-shaped, <90
2 bonds, 3 lone pairs - linear, 180
4 bonds, 0 lone pairs - Tetrahedral, 109.5
3 bonds, 1 lone pair - Trigonal Pyramid, 107.5
2 bonds, 2 lone pairs - bent, 104.5
3 bonds, 0 lone pairs - Trigonal Planar, 120
2 bonds, 1 lone pair - bent, between 104.5 and 120
2 bonds, 0 lone pairs - linear
hybridization - the mixture of atomic orbitals to form a molecular orbital
VSEPR - valence shell electron pair repulsion
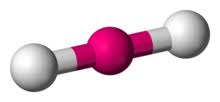
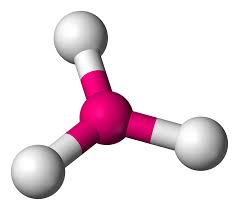
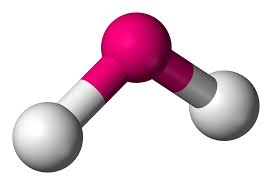
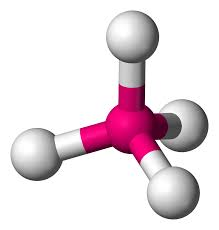
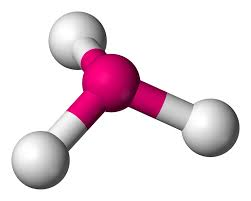
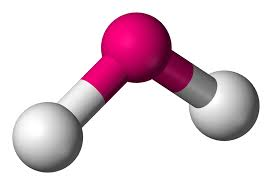
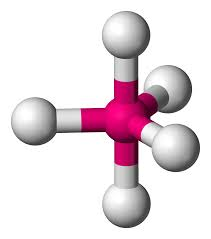
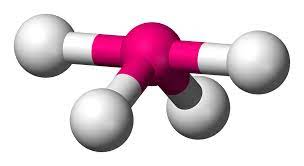
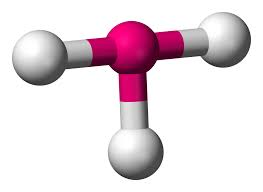
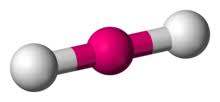
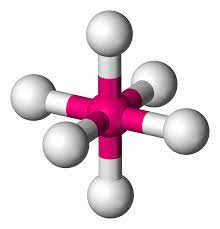
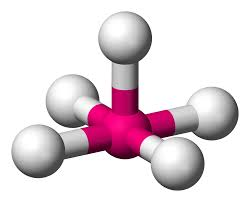
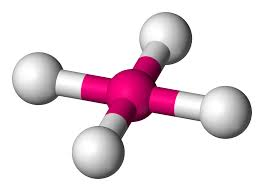
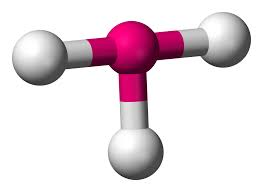
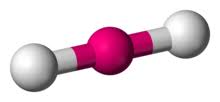
anything with one lone pair - is polar
bent and t shape with 3 lone pairs - is polar
EN difference 0 - covalent non polar
EN difference 0.4 through 1.7 - covalent polar
EN difference <1.7 - ionic