C3 - Chemical Reactions
3.1 Introducing Chemical Reactions
Chemical Reaction Basics
Chemical symbol - The abbreviation used for each element, with the first letter capitalized and the second letter (if present) lowercase. Can be found on the periodic table.
Example: The chemical symbol for zinc is Zn and the chemical symbol for iron is Fe.
Ion - An atom or molecule with a net positive or negative charge as a result of losing or gaining electrons.
A common ion is Na+, a sodium ion that has lost an electron. Sodium typically has one valence electron and Na+ has zero. Many atoms will ionize to gain the stability of Group 18 (noble gas) elements.
Why does the sodium ion have a positive charge? Sodium has 11 electrons and 11 protons, so an atom of Na has a net charge of 0. Na+ is missing an electron, so it has 10 electrons and 11 protons. Its net charge is +1
Polyatomic ions - ions that contain two or more atoms.
Some common polyatomic ions: OH- (hydroxide), CO32-(carbonate), NO3- (nitrate), and SO42- (sulfate).
Diatomic elements - A few elements that typically exist as molecules with two atoms. These are written with a subscript to reflect this. H2, N2, O2, F2, Br2, Cl2, and I2 are all diatomic atoms.
Covalent bond - The bond that forms between two atoms that share electron pairs. Covalent bonds are typically between two nonmetal atoms.
Ionic bond - The bond that forms from the electrostatic attraction between two ions, resulting in the transfer of an electron.
Reactants - The substances that are consumed during a chemical reaction. In a chemical reaction, the reactants are written on the left side of the arrow.
Products - The substances that are produced during a chemical reaction. In a chemical reaction, the products are written on the right side of the arrow.
Electrostatic attraction - The attraction between charged particles (ex. electrons, protons, or ions). This attraction affects the structure of atoms, as well as the way atoms interact.
Mole - A common unit used in chemistry to measure particles, such as atoms and molecules. How many atoms are in a mole of atoms? 6.022 × 1023. (This number is also called the Avogadro constant)
Molar mass - the mass of one mole of a particular substance. Molar mass can be found using the atomic masses of each atom present.
Example: to find the molar mass of HNO3, first use the periodic table to find the atomic mass of each atom.
The molar masses for hydrogen, nitrogen, and oxygen are 1.008 g/mol, 14.007 g/mol, and 15.999 g/mol, respectively.
If there are multiple atoms of one element present: Multiply the element’s molar mass by the number of atoms in the compound. In this case, there are 3 oxygen atoms. 3 x 15.999 = 47.997
Finally, add the total masses together. 1.008 + 14.007 + 47.997 = 63.012 g/mol
Chemical Reaction - The process that involves one or more substances (reactants) undergoing changes to form different substances (products).
The process of hydrogen (H2) and nitrogen (N2) interacting to form ammonia (NH3) is a chemical reaction.
Chemical equation - The symbolic form of a chemical reaction. The general equation is [Reactants] → [Products]. Coefficients are used to denote the number of moles of each reactant or product.
3H2 + N2 → 2NH3 is a chemical equation.
A chemical equation may be written with state symbols. These tell you what physical state each substance is in.
(g) = gaseous state
(s) = solid state
(l) = liquid state
(aq) = aqueous state
A substance is in an aqueous state if it is in a water-based solution.
Balanced chemical equation - A chemical equation that shows the proper ratios of reactants and products. Each side of the equation should have the same amount of each element present.
Stoichiometry - The ratio of the amounts of reactants and products in a balanced chemical equation. A common stoichiometric relationship is the mole ratio, the comparison of the moles used and produced in a reaction.
Ex. To find the ratio between oxygen atoms and water vapor in the equation [ H2 (g) + O2 (g) → H2O (g) ] , the first step is to balance the equation.
Balanced equation: 2H2 (g) + O2 (g) → 2H2O (g)
From the balanced equation, we can see that for every mole of oxygen (O2) used in this reaction, 2 moles of water vapor (H2O) are produced.
1 mol O2 : 2 mol H2O
Steps to Balance an Equation
Look at both sides of the equation and determine if each side has the same number of atoms of each element. If not, the equation is unbalanced.
Balance by changing coefficients: You cannot change the subscripts of any substance in the equation, but you can change the coefficients. Changing the coefficient of one substance to balance one element may cause another coefficient to be changed. Be sure to keep counting the number of atoms of each element on both sides as you go!
Once you confirm the number of atoms of each element is the same on both sides of the equation, the equation is balanced!
If required, make sure to include state symbols when you rewrite the equation.
Example of Balancing an Equation
Step | Equation | Balanced? |
Given: | Fe + Br → FeBr3 | No. On the left side of the equation, there are one Fe atom and one Br atom. On the right side, there are one Fe atom and three Br atoms. |
Multiplying Br by 3 to balance the amount of Br atoms on the right side of the equation | Fe + 3Br → FeBr3 | Yes! There are one Fe atom and three Br atoms on each side of the equation |
Law of Conservation of Mass and Systems
The law of conservation of mass is a concept used in chemistry, stating that mass cannot be created or destroyed. This holds true for the mass of substances in a chemical reaction.
(total mass of the products) = (total mass of the reactants)
A system is what is being looked at in a chemistry problem, including reactants, products, containers, and sometimes the surrounding environment. Reactions can occur in closed or non-enclosed systems.
Closed system - A system in which no substance can enter or escape. This could be a sealed container. Sometimes open-beaker reactions are classified as a closed system if none of the reactants or products are gaseous or otherwise able to escape the container.
Non-enclosed system - A system in which substances can enter or escape. This could be an open container. Reactions in non-enclosed systems typically include a gaseous reactant or product
Equations with Ions
Half equation - an equation that shows the change that one substance undergoes in a chemical reaction.
Example: Ca2+ + 2e- → Ca
Half equations also must be balanced. In a balanced half equation, there are the same amount of each atom on either side of the equation. The net charge also has to be the same on either side.
Complete ionic equation - an equation that shows the ions dissolved from soluble compounds in a chemical reaction, along with the resulting precipitate(s).
Example: Ag+ (aq) + NO3- (aq) + Na+ (aq) + Cl- (aq) → AgCl (s) + Na+ (aq) + NO3- (aq)
Net ionic equation - shows only the reacting ions and the resulting precipitate(s) in a reaction.
Example: Ag+ (aq) + Cl- (aq) → AgCl (s)
Spectator ions - ions that do not participate in the reaction. These are not shown in a net ionic equation.
3.2 Energetics
Temperature Changes in Chemical Reactions
A chemical reaction can be described as endothermic or exothermic. This is based on the way energy is transferred to or from the surroundings while the reaction takes place.
Endothermic: Energy is absorbed and acts as a reactant in the reaction. Temperature of the surroundings decreases.
Example: electrolysis is an endothermic reaction.
Exothermic: Energy is released as a product of the reaction. Temperature of the surroundings increases.
Example: combustion is an exothermic reaction.
Reaction Profile
During a reaction,
Energy is used to break bonds in the reactants
Energy is released when bonds are formed in the products
A reaction is endothermic if more energy is used to break down the bonds in the reactants than is released when forming the bonds of the products.
A reaction is exothermic if less energy is used to break down the bonds in the reactants than is released when forming the bonds of the products.
This can most easily be shown in reaction profile diagrams.
A reaction profile diagram shows:
The energy of the reactants
The energy of the products
Activation energy (EA) - the minimum energy required for a reaction to occur
This is shown as a bump in the graph.
The overall change in energy (the difference between the energy of the products and the energy of the reactants) determines whether the reaction is exothermic or endothermic.
Below is an example of a reaction profile diagram.
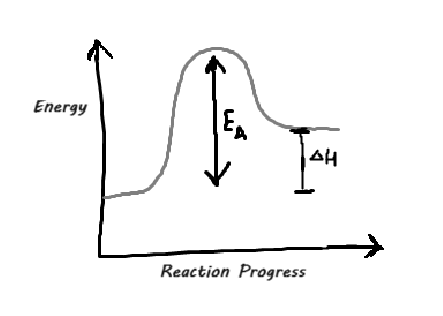
Is the reaction endothermic or exothermic?
Since the energy of the products is higher than the energy of the reactants, this reaction is exothermic.
Remember that there are more reactants than products at the start of the reaction, so the left side of the curve shows the energy of the reactants.
Similarly, there are more products present at the end of the reaction, so the right side of the curve shows the energy of the products.
Calculating Energy Changes
Bond energy - The amount of energy necessary to break one mole of a particular covalent bond
Different bonds require different amounts of energy to break.
unit = kJ/mol
To calculate the energy change for a reaction, you have to find the net energy change. Remember that energy is used to break bonds in the reactants, then released to form bonds in the products.
First, add together the bond energies for all the bonds in the reactants. This energy is used to break down the reactants in the reaction.
Then, add together the bond energies for all the bonds in the products. This energy is released to form the products in the reaction.
Finally, subtract the energy released from the energy used to get the total energy change for the reaction.
Simply: energy change = (energy of the reactants) - (energy of the products)
If the energy change is:
Negative: the reaction is exothermic.
Positive: the reaction is endothermic.
3.3 Types of Chemical Reactions
Included in this section:
Oxidation and reduction
Acidic and alkaline solutions
Neutralization
Reactions of acids
Oxidation and Reduction
Oxidation-Reduction (or Redox) reaction - A reaction in which electrons are transferred between chemical species.
Oxidation and reduction are typically shown as half-reactions. A redox reaction shows the combined reaction of a pair of oxidation/reduction half-reactions.
Example of a redox reaction: 2Na + Cl2 → 2Na+ + 2Cl-
Oxidation - the process of a substance gaining oxygen, or losing electrons, during a chemical reaction.
Example of an oxidation half-reaction: Na → Na+ + e-
Reduction - the process of a substance losing oxygen, or gaining electrons, during a chemical reaction.
Example of a reduction half-reaction: Cl2 + 2e- → 2Cl-
Oxidizing agent - the substance in the reaction that oxidizes the other. This agent also undergoes reduction.
Reducing agent - the substance in the reaction that reduces the other. This agent also undergoes oxidation.
Oxidation number - the number of electrons lost or gained in order for an atom to form a bond with another atom. For some elements, the oxidation number is always the same.
To find the oxidation number of an atom in a substance, use known oxidation numbers and the net charge of the compound. See typical oxidation numbers in the table below:
Element | Oxidation Number | Exceptions |
Group 1 metals | Always +1 | |
Group 2 metals | Always +2 | |
Fluorine | Always -1 | |
Oxygen | Usually -2 | In peroxides. Then, it is -1. |
Hydrogen | Usually +1 | In metal hydrides. Then it is -1. |
Chlorine | Usually -1 | In compounds with O or F. |
Other cases: Pure elements (example: O2) have an oxidation number of zero.
—
Example: Oxidation Numbers
Find the oxidation numbers of each element present in aluminum oxide (Al2O3)
Note the overall charge. In this example, there is no charge. All the oxidation numbers must add up to zero.
Write down all the known oxidation numbers.
We know that the oxygen in this formula has an oxidation number of -2.
Make an equation. Remember to account for the number of atoms for each element (do not ignore the subscript!)
Net charge = 2 (oxidation number for Al) + 3 (oxidation number for O)
0 = 2 (oxidation number for Al) + 3 (-2)
Solve for the missing oxidation number.
0 = 2 (oxidation number for Al) + 3 (-2)
6 = 2 (oxidation number for Al)
Oxidation number for Al = +3
Solution: Aluminum = +3, Oxygen = -2
Acidic and Alkaline Solutions
Solutions contain a solute and a solvent. A solute is the substance that is dissolved and a solvent is the substance that the solute dissolves into. For example, if you dissolve salt in water, salt is the solute and water is the solvent.
Acid - substance that produces an acidic solution in water. Acids produce more hydrogen ions (H+) than hydroxide ions (OH-) when dissolved.
Alkali - substance that produces an alkaline solution in water. Alkalis produce more hydroxide ions than hydrogen ions when dissolved.
pH scale - measures the acidity (or alkalinity) of a particular solution
pH < 7 → acidic
pH = 7 → neutral
pH > 7 → alkaline
How is pH measured?
pH can be measured using universal indicator, a chemical solution that changes color based on pH level.
To use: add one drop of solution to a piece of universal indicator paper. Once the color develops, match it to the color chart provided with the indicator paper. An acidic solution turns indicator paper red, a neutral solution turns it green, and an alkaline solution turns it blue.
The color chart shows a range of colors, so an acidic solution with pH = 0 will turn the indicator paper dark red, while a solution with pH = 4 will appear orange.
Another way to measure pH is through a pH meter.
To use: place the end of the probe into the solution and record the reading on the pH meter.
Neutralization
Base - a substance that can neutralize an acid and produce a salt as a result.
All alkalis are bases, but not all bases are alkalis. Alkalis are soluble bases, but some bases are insoluble.
Neutralization reaction - A reaction that involves an acid and a base or an alkali. The resulting products are a salt and water.
In general: acid + base/alkali → salt + water
Important to note: acids in solution form H+ and alkali solutions contain OH-. A neutral solution (pH = 7) can be formed if the right amounts of acid and alkali are present in the reaction.
Reactions of Acids
Reactions with metals: when acids and metals react, a salt and a hydrogen form.
In general: acid + metal → salt + hydrogen
Example: H2SO4 (aq) + Zn (s) → ZnSO4 (aq) + H2 (g)
Reactions with carbonates: when acids and carbonates react, a salt, water, and carbon dioxide form.
In general: acid + carbonate → salt + water + carbon dioxide
Example: 2HCl + CuCO3 → CuCl2 + H2O + CO2
How to predict what salt is formed?
A salt is named based on the metal and the acid involved. Look at the components. Remember that these reactions involve dissociated ions. When H+ and OH- react, H2O forms. So what is left?
For example, calcium phosphate forms when calcium carbonate reacts with phosphoric acid.
Acid Concentration and Strength
The concentration of a solution is a measure of how many particles of solute are present in a given volume.
A dilute solution has a low solute:solution ratio
A concentrated solution las a high solute:solution ratio
Strong acids completely dissociate in solution. Some strong acids are HCl, HNO3, and HBr
Weak acids only partially dissociate in solution.
pH and H+ Concentration
pH = -log[H+], where [H+] is the concentration of hydrogen ions.
The higher the concentration of H+ ions in an acidic solution, the lower the pH (and the more acidic the solution is).
To find the pH of an alkaline solution, remember that solutions with high OH- concentrations have low H+ concentrations, and vice versa. Since alkaline solutions have high OH- concentrations, their pH is above 7.
As a general rule, the higher the concentration of OH- ions, the higher the pH of a solution.
3.4 Electrolysis
Electrolytes - ionic compounds that are either dissolved in water or molten
Molten substances are liquid, formed by adding heat to a solid
In either of these states, ions are free to move throughout the liquid.
Electrolysis - the process of breaking down electrolytes using an electric current. The resulting free ions are attracted to the charged electrodes connected to the source of the current.
Electricity is the flow of electrons.
Cations - positively charged ions
Example: Na+, H+, Mg2+
Anions - negatively charged ions
Example: Cl-, OH-, SO42-
Electrode - A conductor connected to the source of the current and submerged in the electrolyte substances to create a circuit. There are two oppositely charged electrodes.
Cathode - the negatively charged electrode that attracts positively charged ions (cations)
Anode - the positively charged electrode that attracts negatively charged ions (anions).
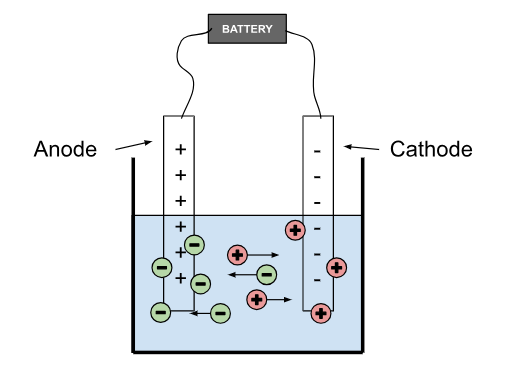
Products of Electrolysis
Cations go to the cathode, and anions go to the anode. When ions get in contact with electrodes, they form different molecules.
When cations (+) reach the cathode (-), they gain electrons
Example: At the cathode, Na+ would become Na
When anions (-) reach the anode (+), they lose electrons
Example: At the anode, Cl- would become Cl
Example: In the electrolysis of molten aluminum oxide, what products are formed at the electrodes?
Molten aluminum oxide has freely flowing aluminum ions (Al3+) and oxide ions (O2-). The aluminum ions move towards the cathode and gain electrons, while the oxide ions move towards the anode and lose electrons.
Answer: Aluminum forms at the cathode and oxygen forms at the anode.
Electrolysis of Acidified Water
Water contains H+ and OH-. If water is acidified (mixed with a small amount of acid), the H+ ions go to the cathode and form hydrogen gas (H2). The OH- ions go to the anode and form oxygen gas (O2).
Remember that at the electrodes, anions lose electrons and cations gain electrons.
Electrolysis of Dissolved Ionic Compounds
A dissolved ionic compound solution contains H+ and OH- ions from the water, as well as anions and cations from the compound. The ions compete to gain/lose electrons at the electrode.
If the metal cation is less reactive than hydrogen, the metal will form at the cathode. If the metal cation is more reactive than hydrogen, then hydrogen will form.
Refer to “Reactivity Series of Metals” below
Oxygen is produced at the anode unless the dissolved compound contains halide ions (Cl-, Br-, I-).
Reactivity Series of Metals (most to least reactive)
Hydrogen shown for comparison
K
Na
Li
Ca
Mg
Al
Zn
Fe
H
Cu
Ag
Au
Electroplating
Electroplating - the use of electrolysis to put a thin layer of metal onto another metal object. In this case, the electrodes used take place in the reaction. Electroplating can be used to keep metal objects from corroding.
Parts:
The cathode is the object to be plated
The anode is the metal that forms the plated layer
The electrolyte contains the ions of the plating metal
Electroplating can also be used to purify copper
Cathode: pure copper
Anode: impure copper
Electrolyte: copper(II) sulfate solution (contains Cu2+ ions)