Chemical Changes: Making Salts (Practical)
Preparation of pure dry copper sulfate crystals
In this investigation you will use the reaction between an acid and an insoluble base to prepare an aqueous solution of a salt. After filtering to remove excess unreacted base, you will evaporate the filtrate to leave a concentrated solution of the salt, which will crystallize as it cools and evaporates further. These crystals, when dry, will be of high purity.
EQUIPMENT
40 cm3 1.0M dilute sulfuric acid
Copper (II) oxide powder
Spatula
Glass rod
100 cm3 beaker
Bunsen burner
Tripod, gauze
heatproof mat.
Filter funnel and paper
Clamp stand
Conical flask.
250 cm3 beaker
evaporating basin
crystallizing dish.
risk assessment: safety goggles must be worn throughout!
INSTRUCTIONS
1) Measure 40 cm3 sulfuric acid into the beaker. The volume does not need to be very accurate, so you can use the graduations on the beaker.
2) Set up the tripod, gauze and heatproof mat. Heat the acid gently using the Bunsen burner until it is almost boiling. Turn off the burner.
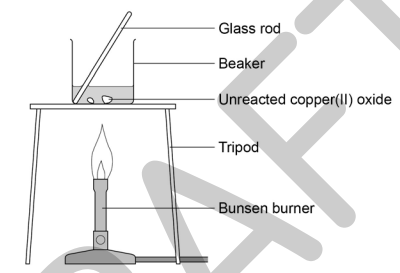
3) Using the spatula, add small amounts of copper (II) oxide powder at a time, stirring with the glass rod. Continue to do this if, after stirring, the black powder disappears and the solution is clear blue.
4) Stop adding it when some black powder remains after stirring.
5) Set up the filter funnel and paper over the conical flask, using the clamp stand to hold the funnel. Filter the contents of the beaker from step 3.
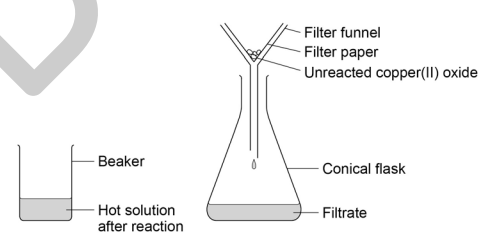
6) When filtration is complete, pour the contents of the conical flask into the evaporating basin. Evaporate this gently using a water bath on the tripod and gauze (see diagram) until around half of the solution remains. You will have to estimate this volume.
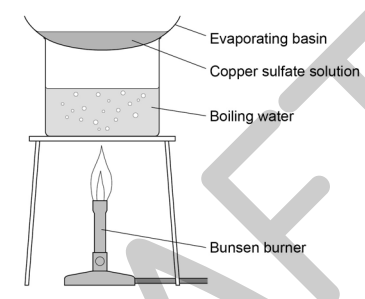
7) Transfer the remaining solution to the crystallizing dish. Leave this in a cool place for at least 24 hours.
8) Remove the crystals from the concentrated solution with a spatula and gently pat them dry between two pieces of filter paper. These are pure dry crystals of copper (II) sulfate.
60 Copyright © 2015 AQA and its licensors. All rights reserved.